Fig. 17.1
Typical MRI appearance of large sacral chordoma
17.3 Clinical Features
Patients diagnosed with a chordoma most commonly present with pain, regardless of location (Bergh et al. 2000; Boriani et al. 1996, 2006). The second most common presentation is development of neurological symptoms and least commonly a palpable mass (Bergh et al. 2000, Soo 2001). Symptom duration averages 2 years prior to diagnosis, highlighting the slow-growing nature of these tumors (Bergh et al. 2000). If untreated, pain can progress to the point of incapacitation, which in one study was found to be at approximately 50 months from the onset of symptoms (Boriani et al. 2006).
Up to 60 % of the time, chordomas can extend into the spinal canal and in some of these cases can cause significant neurologic symptoms, such as compressive myelopathy or cauda equina (Meyer et al. 1984) (Fig 17.2). Neurological symptoms are most commonly associated with chordomas of the mobile spine. The spectrum of neurological symptoms widely ranges depending on the location of the tumor. Severe compression of the spinal cord is a late complication leading to paralysis. Tumors which invade the neural foramina can cause a radicular pattern of symptoms, which includes weakness and sensory deficits in the distribution of a particular nerve root (Sundaresan et al. 1990; Mindell 1981).
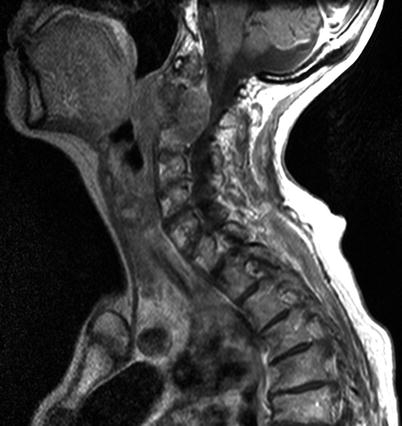

Fig. 17.2
Large cervical spine chordoma at C2 with compression of spinal cord
Non-neurologic symptoms from chordoma are most commonly due to local effects of the tumor mass. For example, chordomas which develop in the cervical spine may cause throat irritation, dysphagia, esophageal compression, dysphonia, or airway obstruction from compression of critical local structures (Singh et al. 2007; Nicoucar et al. 2008). Horner syndrome has been reported from lower cervical spine chordomas (Leone et al. 2002). Mass effect from of sacral chordomas can cause compression and displacement of the bladder or rectum leading to urinary stress incontinence, constipation, or obstruction. Sacral chordoma of sufficiently large size can be palpated on rectal exam (Fourney and Gokaslan 2003; Atalar et al. 2006).
17.3.1 Box 17.1 The Cauda Equina
Anatomically, the cauda equina, or “horse’s tail,” is the collection of nerve roots that travels through the spinal canal beyond the termination of the spinal cord. The spinal cord ends at approximately the L1 level in humans, at which point the lumbar (L1–5) and sacral (S1–5) roots have already branched off the spinal cord. However, because these nerve roots exit the spinal canal at successively more inferior levels in the lumbar and sacral bony spine, they travel together for a distance within the spinal canal as a bundle of nerve roots, which is called the cauda equina. Injuries or other conditions which damage the cauda equina cause a specific constellation of symptoms related to dysfunction of these critical nerve roots and is considered a surgical emergency.
Symptoms of cauda equina syndrome from any cause include weakness in the lower extremities, urinary retention due to detrusor muscle weakness, loss of rectal tone due to anal sphincter weakness, and subsequent fecal incontinence. Sexual dysfunction and saddle anesthesia and lower extremity pain may also be present. Lower extremity reflexes are reduced or absent. The causes of cauda equina syndrome are numerous and essentially can be any inciting agent or problem which causes pressure on the cauda equina. Commonly, acutely herniated discs can cause cauda equina syndrome. Other degenerative spinal conditions such as spinal stenosis or spondylolisthesis can contribute as well. Trauma is another common cause, either by direct damage to nerve roots by fracture, dislocation, or penetrating trauma or by hematoma secondary to the initial traumatic injury. Tumors, such as chordoma or more commonly metastatic disease, can also cause a cauda equina syndrome, although in this situation the presenting symptoms are usually more chronic and develop over a longer period of time as the tumor grows.
Treatment of cauda equina syndrome is primarily surgical decompression of the involved nerve roots as well as removal or correction of the inciting mechanical problem. In the case of degenerative and traumatic conditions, every effort is made to preserve the involved nerve roots. In the case of metastatic tumors, intralesional procedures may be performed in conjunction with adjuvant therapies such as radiation. Sacral chordoma requires wide resection, which usually involved resection of some or all of the sacral nerve roots, so the expectation is that the nerve deficits will not recover. It is important to discuss the specific expected deficits with any patient undergoing sacrectomy for chordoma.
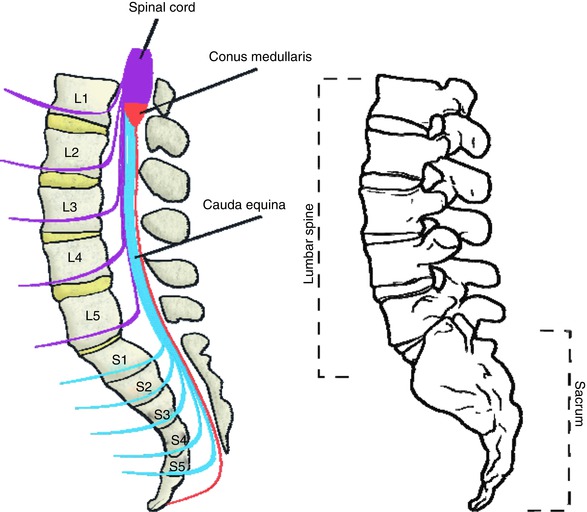

The cauda equina is shown schematically on the left, and the osseous anatomy of the lumbar spine and sacrum is shown on the right
17.4 Tumor Imaging
Plain radiographs are often the initial image taken for a complaint of back pain. However, detailed examination of the spine with plain imaging alone can be difficult. In particular, the obliquity of the sacrum and the overlying shadows from bowel gas and contents can often limit its utility (Manaster and Graham 2003). Nonetheless, when evaluating the sacrum on plain radiographs, there are particular features that should be scrutinized: the paired sacral foramen should appear similar with distinct sacral boundaries outlining the foramen, the anterior and posterior aspects of the sacroiliac joint should be distinct, and the posterior contour of the iliac wing should be seen underlying the sacral ala. Lack of any of these findings on pelvic radiograph could suggest a lesion of the sacrum. Intratumoral amorphous calcifications are seen on plain radiographs within a chordoma in 50–70 % of cases and in 90 % of cases on CT scan, but have no known prognostic significance (Fig 17.3). An associated large soft tissue mass can also be seen on CT scan or MRI.
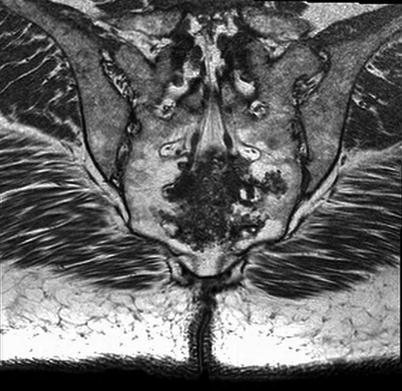

Fig. 17.3
Coronal MRI image of sacral chordoma showing extensive calcifications, sometimes seen in chordoma
Evaluation of the central spinal canal and cord or nerve root involvement is best done with MR imaging, and as such this modality is critical for evaluation of chordomas. MRI features of a chordoma on T1-weighted sequences consist of an isointense or hypointense mass as compared to muscle and on a T2-weighted sequences as a high-signal-intensity mass (Fig 17.4). If calcifications are present, they can appear as areas of low signal intensity on T1- and T2-weighted images. Chordomas enhance with gadolinium (Manaster and Graham 2003). Primary tumors and metastatic lesions both show very high signal intensities on diffusion weighted images, which may help distinguish metastatic nodules from nodules of other unrelated etiologies (Kishimoto et al. 2012). Both MRI and CT scan can show bone destruction and extension of the tumor into the canal (Fig. 17.5). MR imaging can be used to differentiate benign notochordal cell tumors (BNCTs) from chordomas based on the lack of gadolinium uptake, bone sclerosis, and completely intraosseous location seen with BNCTs (Nishiguchi et al. 2011). Chordomas also demonstrate fluorodeoxyglucose avidity on F-18 PET scans (Lin et al. 2006; Miyazaway et al.2008; Park and Kim 2008). Carbon-11-methionine positron emission tomography (MET-PET), used to evaluate the effectiveness of carbon-ion radiotherapy for assessment of rectal cancer and other tumors, has been used to study chordoma treatment. This technology has shown some promise in pre- and posttreatment imaging of chordoma (Zhang et al. 2004).
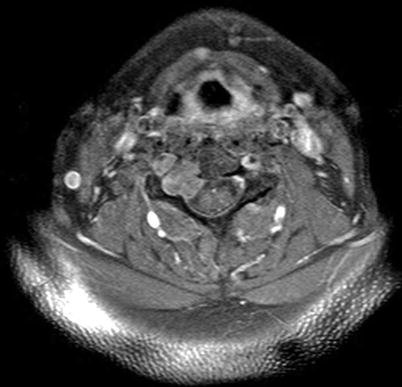
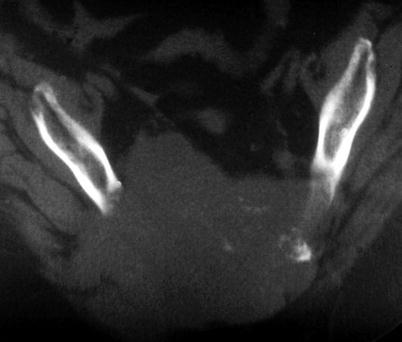

Fig. 17.4
Typical MRI appearance of cervical spine chordoma, in this case involving right-sided pedicle

Fig. 17.5
Axial CT scan image showing extensive sacral bone destruction due to chordoma. Note that the tumor is a midline tumor, which can differentiate it from other sacral tumors
17.5 Histopathology and Immunohistochemistry
Virchow’s original description of the physaliphorous cell, a vacuolated cell clustered in sheets giving the appearance of “soap bubbles” and demonstrating a lobular pattern of growth, describes the classic histologic features of chordomas (Fig. 17.6). These cells have small round dark staining nuclei with few mitotic figures but demonstrate significant atypia. The sheets of cells are separated by a fibrous septae with areas of calcification or hemorrhage representing necrotic areas (Weber and Sim 2002). These tumors can be separated into three classes: classical or conventional, chondroid, and dedifferentiated (Chugh et al. 2007). The classical form most commonly displays the typical physaliferous features. Chondroid type tumors may exhibit areas of chondrosarcoma-like cartilage as well as features of classic chordoma. Dedifferentiated tumors may have a more highly aggressive sarcomatous histological appearance. Dedifferentiated tumors may display nuclear inclusions, bi- or multinucleation, and sometimes mitotic figures (Crapanzano et al. 2001). Immunohistochemical staining is useful in the evaluation of chordomas. Significant immunoreactivity for S-100, membrane antigen (MUC-1), and cytokeratin is seen in these tumors.
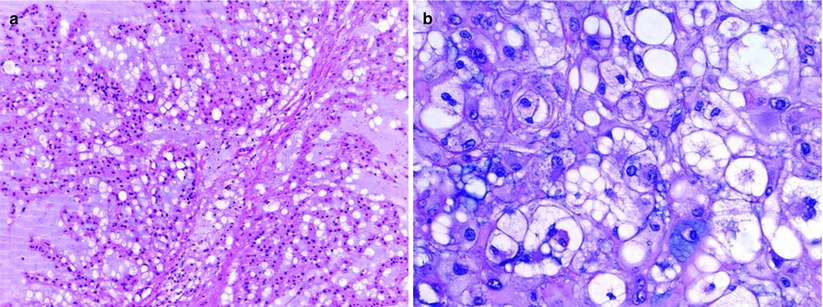

Fig. 17.6
Photomicrograph of chordoma histology demonstrating vacuolated physaliferous cells. (a) Low power, 100×; (b) high power, 400×
Chondroid tumors are difficult to distinguish from chondrosarcomas, emphasizing the need for specific immunohistochemical markers. Brachyury is a notochordal transcription factor that is expressed in most sporadic chordomas, but not in chondrosarcomas (Vujovic et al. 2006). Interestingly, the T gene locus containing the brachyury gene has been found to be duplicated in rare familial instances of chordoma, suggesting a critical role in the pathogenesis of this tumor (Yang et al. 2009). The addition of brachyury to the panel of biomarkers used to identify this cell type has improved the sensitivity and specificity for chordoma to 98 and 100 % (Oakley et al. 2008). Other important biomarkers such as ezrin, MMP-9, and COX-2 have also been recently investigated and hold diagnostic promise (Froehlich et al. 2012). The next step in the investigation of these markers is to study their value as potential therapeutic targets.
17.6 Differential Diagnosis
In the differential diagnosis of tumors of the spine, myeloma, plasmacytoma, benign notochordal cell tumor, lymphoma, osteomyelitis, giant cell tumor, and chondrosarcoma need to be considered (Sciubba et al. 2009). Key clinical, radiographic, and histologic findings help distinguish each type of tumor from a chordoma. Plasmacytoma may have a similar radiographic appearance as chordoma. A positive scintigraphy seen with a chordoma may differentiate these tumors (Greenspan et al. 2006). Radiographically, osteomyelitis and lymphoma can be difficult to distinguish from a chordoma; however, their clinical course and laboratory data can usually be used to distinguish these conditions (Sciubba et al. 2009). Benign notochordal cell tumors are usually asymptomatic, may demonstrate a more sclerotic appearance, and have no associated soft tissue mass. Giant cell tumors are benign but locally aggressive tumors that are often seen in the sacrum and can have an appearance similar to that of a chordoma. Chordomas have more of a midline predilection, however, and giant cell tumors often demonstrate a thin rim of peripheral bone encompassing the soft tissue mass which is not typically seen in a chordoma. Chondrosarcoma and chondroid chordomas can have similar appearances, and the biomarkers discussed above therefore play a critical role in distinguishing these tumors.
17.7 Tumor Staging
Staging is the process of defining the local and distant extension of a cancerous disease process. In the case of chordoma, MRI is the primary imaging modality used to evaluate the location and extent of the tumor. The presence of metastatic disease is evaluated using CT scan of the chest, abdomen, and pelvis with intravenous and oral contrast agents. Nuclear medicine bone scintigraphy can show other skeletal lesions. PET metabolic imaging can be used to demonstrate fluorodeoxyglucose avidity at sites of disease, but has some limitations based on lesion size, and is not approved as a first-line modality for this purpose. F-18 PET or MET-PET are potentially promising techniques as described earlier.
17.8 Management
17.8.1 Surgery
The primary treatment of chordoma is wide surgical resection (Fig. 17.7) and the goal of surgery is wide excision with negative margins. This is sometimes an unrealistic goal due to the difficult locations of this tumor, especially clival or skull-base tumors, or high-level sacral tumors. Nonetheless, multiple studies have demonstrated a correlation between recurrence rate and positive margins (Boriani et al. 2006; Bilsky et al. 2004; Fuchs et al. 2005). Boriani et al. (2006) demonstrated that in the absence of negative margins, the recurrence rate was approximately 70 and 100 % following radiation treatment alone, palliative care, or intralesional excision. When wide excision with adequate margins was obtained, there was a recurrence rate of 20 % diagnosed at 56–94 months post surgery. In a group of patients all treated with en bloc excision for sacrococcygeal chordoma, Fuchs et al. (2005) demonstrated an overall survival rate of 74 % at 5 years, 52 % at 10 years, and 47 % at 15 years. Interestingly, the survival rate in this group of patients was significantly higher when negative margins were obtained, and the most significant predictor of survival was a wide margin. The size of the tumor, level of resection, and surgical approach did not significantly impact the survival rate.
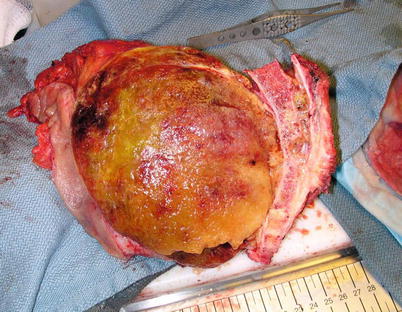

Fig. 17.7
Intraoperative photograph showing sacral chordoma after resection
Sacral resection can be achieved using either a combined anterior-posterior approach or a posterior-only approach. Generally, for tumors with significant anterior soft tissue mass, or high sacral resections, there is a benefit to first performing an anterior dissection prior to resection from a posterior approach. This was best shown by Fuchs et al. (2005) in a series from the Mayo Clinic in which 81 % of patients with combined anterior-posterior approaches had negative margins. Based on these results, it was recommended that a combined dual approach should be used for any patients with tumors above S3.
After chordoma resection, functional deficit is dependent on the extent of the surgery. To maximize survival rates and to prepare patients for postoperative deficits, careful preoperative planning and discussion of possible neurological problems is important. Whether partial or total sacrectomy is performed, usually one or more sacral nerve roots will need to be sacrificed, leading to a motor deficit, sensory impairment, sphincter loss, and/or sexual dysfunction. Fourney et al. (2005) developed a classification system describing the type of sacrectomy based on the level of nerve root sacrificed, as opposed to the site of the osteotomy. The type of resection was defined as low, middle, high sacral amputation, total sacrectomy, or hemicorporectomy. Sacral amputations are considered “low” if at least one S4 nerve root is sacrificed, “middle” if at least one S3 nerve root is sacrificed, and “high” if at least one S2 nerve root is sacrificed. If neither S1 nerve root can be spared, then the required amputation is a total sacrectomy. A hemicorporectomy (translumbar amputation) is performed when the tumor extends to the lumbar spine. Functionally, if both S2 roots can be spared, half of the patients or more will have normal bowel and bladder function. If an S3 root is preserved as well, these odds improve. However, perineal numbness and sexual dysfunction are still commonly observed, the latter more common in the elderly. If one S2 root is sacrificed, typically some amount of voluntary control is compromised. Sacrifice of one S1 or S2 nerve root and all lower roots, as seen in high sacral amputations, commonly leads to urinary or fecal incontinence leading to the possibility for the need of indwelling urinary catheters, intermittent straight catheterization, colostomy, or digital stimulation to defecate (Hulen et al. 2006). Loss of ankle plantar flexion is also seen after the loss of the S1 nerve root.
If resection of the lumbar spine or pelvis is required at the time of sacral amputation, spinopelvic instability must be assessed. If instability is observed, then instrumentation is warranted (Fig. 17.8). In general, about 50 % of the sacroiliac joint can be removed before instability is seen, provided the ligamentous structure of the remainder of the joint is preserved (Gunterberg et al. 1976; Stener and Gunteberg 1978). Chordomas of the mobile spine are also best treated with resection with negative margins. To achieve this goal, a total spondylectomy is the surgery of choice and is generally done through a combined anterior and posterior approach (Boriani et al. 2006).
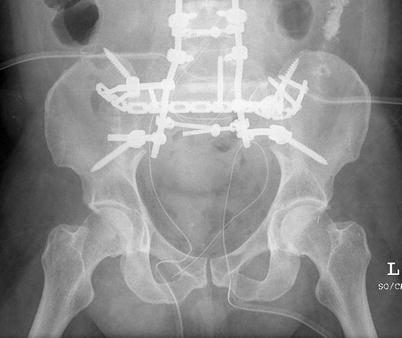

Fig. 17.8
An example of a postoperative reconstruction after resection of entire sacrum
In the skull base, despite multiple surgical approaches, wide resection surgery is rarely possible (Singh et al. 2010; Holzmann et al. 2010). Nonetheless, radiation therapy is effective in managing microscopic or limited gross amounts of residual tumor (Potluri et al. 2011). For this reason the recommended approach in these difficult anatomic areas is aggressive near-total intralesional excision that maximally preserves neurologic function.
17.8.2 Radiation
Radiation therapy has become increasingly important in the management of chordomas. As a result of advances in radiation techniques and modalities, it is now possible to deliver higher doses to the tumor while sparing critical surrounding structures. The limiting factor is the tolerance of the spinal cord, in particular at its upper end, which is lower than the dose needed to effectively treat the tumor. In general, conventional external beam radiation alone, at doses of 40–60 Gy, is suboptimal for treatment of chordoma, resulting in 5-year local control of 10–40 % (Catton et al. 1996; Cummings et al. 1983). Doses of up to 80 Gy have a high rate of associated radiation-induced myelopathy.
Use of high-dose protons and charged particles such as carbon, helium, or neon ions (collectively categorized as hadrons) enables higher doses to be delivered to tumors with limited radiation damage to critical surrounding structures. Indeed, as proton therapy has no measurable exit dose, peripheral structures are spared. Proton beam treatment of chordomas, either alone or in combination with photons, when used in conjunction with primary resection surgery, has proven to be an excellent method for local control (Hug et al. 1999; Noël et al. 2001; Fuji et al. 2011). This is particularly relevant to skull-base tumors since the tolerance is lower than in the peri-sacral regions. In the sacrum, primary surgery and radiation provide better results when compared with treating chordoma reoccurrence, thereby supporting a role for this approach as an effective first-line treatment option (Park et al. 2006).
Carbon-ion radiotherapy has also been studied in chordoma management. Since carbon ions are heavier than protons, this approach is thought to provide a higher biological effectiveness. Interestingly the effectiveness increases with depth, reaching its peak at the end of the beam’s range. This is an extremely attractive property for local control of cancer and as such has led to significant use of carbon-ion therapy in the management of chordoma. 5-year local control rates of 70–88 % and 10-year local control rates of 80–82 % have been reported in skull-base tumors using carbon-ion therapy (Schulz-Ertner et al. 2007; Mizoe et al. 2009; Tsujii and Kamada 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








