CHAPTER 41 Cervical Spondylotic Myelopathy
Surgical Management
Aging is associated with the development and progression of degenerative changes within the cervical spine. Degenerative changes are manifested in the cervical spine as disc height loss, facet and uncovertebral joint osteophytes, spondylotic bars, and hypertrophic ligamentum flavum.1,2 Neural compression from these structures may lead to the development of clinical symptoms consistent with the diagnosis of myelopathy. Although older patients may present with some degree of developmental stenosis from superimposed degenerative changes, younger patients may present with cord compression secondary to a large disc herniation. Although there is still debate regarding the etiology and pathophysiology of neuronal damage that occurs in the presence of spinal stenosis, direct mechanical compression and indirect vascular ischemia have been suggested as potential causes.3 In a cadaveric study, Ono and colleagues3 observed damage to gray and white matter, with cord atrophy, demyelination, and vascular infarction found in several segments removed from the site of maximal cord compression.
The clinical findings early in the natural course of cervical spondylotic myelopathy (CSM) are often very subtle, and delays in diagnosis are common owing to the insidious onset of these symptoms.4–8 Sadasivan and colleagues9 reported an average delay in diagnosis of approximately 6 years with gait disturbances manifesting as the earliest symptom in patients in his series. Often patients subjectively complain of a useless hand, and they notice a decline in fine motor skills. They also observe deterioration in penmanship and dexterity. Patients exhibit a loss of dexterity from atrophy of the thenar and hypothenar eminences, weakness of the extensors of the wrist, and loss of apposition of the thumb. Gait and balance disturbances from proximal lower extremity weakness are noticed and are often attributed to old age.1,10,11 Other signs of myelopathy include hyperactive deep tendon reflexes, clonus, pathologic reflexes such as bilateral Babinski responses, loss of proprioception and stereoanesthesia, and decreased pinprick and vibratory appreciation. Rarely, severely affected individuals may present with frank paralysis affecting bowel and bladder control.
Natural History
The natural history of CSM has been studied extensively in the literature. Clark and Robinson12 followed 120 patients with cervical spondylosis and myelopathy in an effort to describe the natural progression of the disease. These authors noted that of 120 patients, 5% showed a rapid onset of symptoms followed by long periods of remission, 20% showed a slow gradual decline in function without any periods of remission, and 75% showed stepwise deterioration in function followed by episodic periods of remission. In 1963, Lees and Turner13 documented their experience in the treatment of cervical myelopathy. Most patients in their series were noted to have periods of progression of disease mixed with static periods of unchanged symptoms. They also observed that 14 of the 15 patients in their series who presented with severe myelopathy continued to be disabled at 10 to 20 years of follow-up.
Nurick14,15 published his series on the natural history of cervical myelopathy following 36 patients who were treated nonoperatively. In patients who presented with mild clinical symptoms, he observed no significant clinical worsening of their condition at final assessment decades later. In addition, he noted that patients who presented with clinical symptoms at an older age tended to have a worse decline in functional status. The patients with the worst prognosis were patients who presented with severe disability, especially if they were of advanced age. Nurick15a also established a grading system for myelopathy that revolved around the patient’s ambulatory status (Table 41–1). Most series that document the long-term follow-up of patients with CSM report a progression of disease with a gradual deterioration in functional status over time.16–18
TABLE 41–1 Disability Classification of Cervical Spondylotic Myelopathy
| Grade | Description |
|---|---|
| 0 | Root signs and symptoms, no cord involvement |
| I | Signs of cord involvement, normal gait |
| II | Mild gait involvement, able to be employed |
| III | Gait abnormality prevents employment |
| IV | Able to ambulate only with assistance |
| V | Chair-bound or bedridden |
Indications for Surgical Intervention
As documented in natural history studies, patients presenting with moderate to severe symptoms are unlikely to experience regression of myelopathy. To alter the natural progression of the disease and prevent further neurologic deterioration, surgical intervention should be considered. In a review of surgical indications for cervical myelopathy, Law and colleagues19 identified several poor prognostic factors for conservative treatment, including progression of symptoms, presence of myelopathy for more than 6 months, compression ratio approaching 0.4 indicating flattening of the cord, and transverse area of the cord less than 40 mm2. The presence of any of these factors is an indicator for surgical intervention.
The goals of operative intervention include decompression of the spinal cord, stabilization of the spinal column, and reestablishment of the normal sagittal alignment. Preoperative findings that favor a successful surgical outcome include young age at presentation, duration of symptoms less than 1 year, presence of a Lhermitte sign, involvement of pathology limited to fewer vertebral segments, and presence of unilateral symptoms.20 A constellation of findings contributes to the surgeon’s decision to proceed with surgery. Factors that play a role in the decision-making process include duration of symptoms, degree of spinal cord dysfunction, general health of the patient, degree of functional deterioration, and radiographic findings.
The degree of spinal cord dysfunction is evaluated by looking for balance deficits, gait abnormalities, motor weakness, long tract signs, and changes in function. Studies by Okada and colleagues21 and Bohlman22 used clinical symptoms such as gait disturbance as the primary indication for operative treatment. Wada and colleagues23 recommended using a combination of the patient’s clinical findings, preoperative functional status as measured by the Japanese Orthopaedic Association (JOA) scale, and radiographic findings to determine the need for operative intervention. They recommended surgery when symptoms such as gait disturbances and loss of fine motor control were present in combination with a JOA score of less than 13 and radiographic evidence of spinal cord compression. In most reported studies, the neurologic results of laminoplasty are graded according to the JOA myelopathy score. A maximum score of 17 reflects normal function, and the recovery rate describes the extent to which the score returns to normal postoperatively (Table 41–2).
TABLE 41–2 Criteria for Evaluation of Operative Results of Patients with Cervical Myelopathy by Japanese Orthopaedic Association
| Function | Score (Maximum = 17) | Remarks |
|---|---|---|
| Motor | ||
| Upper extremity | 4 | Normal |
| 3 | Able to feed self with chopsticks regularly, but slightly awkwardly | |
| 2 | Able to feed self with chopsticks regularly, although awkwardly | |
| 1 | Able to feed self with a spoon, but not with chopsticks | |
| 0 | Unable to feed self with either spoon or chopsticks | |
| Lower extremity | 4 | Normal |
| 3 | Able to walk on level surface or climb stairs without cane or support but awkwardly | |
| 2 | Able to walk on level surface without cane or support but unable to climb stairs without either of them | |
| 1 | Needs cane or support even when walking on level surface | |
| 0 | Nonambulatory | |
| Sensory | ||
| Upper extremities | 2 | Normal |
| 1 | Slight sensory loss or numbness | |
| 0 | Definite sensory loss | |
| Lower extremities | 2 | Normal |
| 1 | Slight sensory loss or numbness | |
| 0 | Definite sensory loss | |
| Trunk | 2 | Normal |
| 1 | Slight sensory loss or numbness | |
| 0 | Definite sensory loss | |
| Bladder | 3 | Normal |
| 2 | Mild dysuria | |
| 1 | Severe dysuria | |
| 0 | Complete retention of urine | |
Multiple studies have shown that the degree of neurologic recovery depends highly on the preoperative duration of symptoms and the severity of the myelopathy at the time of intervention.24–27 Irreversible histologic and physiologic changes such as intraneural fibrosis and demyelination can occur within the spinal cord with prolonged compression.28 Tanaka and colleagues24 observed that the preoperative duration of symptoms strongly influenced the degree of functional recovery after surgical decompression. They recommended early surgical intervention in the treatment of CSM. Similarly, Suri and colleagues25 noted that patients with symptoms of less than 1 year’s duration showed a greater degree of postoperative motor recovery compared with patients with symptoms of longer duration. Patients with severe forms of myelopathy tend to experience less relative recovery compared with patients with mild symptoms. Bernard and Whitecloud29 also observed that patients with severe preoperative disability and a longer duration of symptoms had poorer outcomes after decompression.
The influence of age on neurologic recovery has also been investigated. Although most patients experience significant neurologic improvement after decompression, the degree of recovery tends to be mildly improved for younger age groups.21,24,26,27 Hasegawa and colleagues30 believed that this discrepancy occurred because elderly patients had a higher incidence of new neurologic dysfunction arising from different sources.
Radiographic studies also play an important role in the operative management of CSM. The normal mid-sagittal spinal canal diameter measures 17 mm in depth, and the normal spinal cord measures approximately 8 to 13 mm in its anteroposterior dimension.31,32 The addition of soft tissue structures, including the posterior longitudinal ligament and the ligamentum flavum, can occupy an additional 2 to 3 mm of the canal diameter.33 Patients with narrowing of the canal to 13 mm are considered to have relative stenosis, whereas patients with narrowing of 10 mm are considered to have absolute stenosis.34 Sites of maximal cord compression may manifest as high signal intensity on T2-weighted magnetic resonance imaging (MRI). These hyperintense signals in the cord may represent intraspinal edema or neuronal death and are generally referred to as myelomalacia. Evidence of myelomalacia on MRI has been associated with a greater degree of clinical disability and a poor prognostic finding for neurologic recovery after surgery.35,36 Suda and colleagues37 found that high cord signal intensity on MRI was associated with inferior surgical outcomes.
Radiographic evidence of dynamic stenosis as measured by translation between the vertebral bodies or by shingling of the lamina in hyperextension can transiently narrow the spinal canal.38 These structural changes in conjunction with the associated buckling of the ligamentum flavum during hyperextension of the cervical spine may decrease further the space available for the cord and may precipitate the development of myelopathy.39 Penning40 showed that cervical myelopathy should be strongly suspected if the canal space measured on dynamic lateral radiographs is reduced to less than 11 mm.
When the decision for operative intervention is made, the surgeon is faced with different approaches and operative techniques for the surgical treatment of cervical myelopathy. Regardless of whether a posterior or an anterior approach is used, the primary goal of surgical intervention in the treatment of CSM is to decompress the spinal canal. Appropriate expansion of the spinal canal has been shown to improve cord morphology and likely to maximize blood flow to the cord.41,42 Important factors that may influence the choice of approach include the sagittal alignment of the spinal column, the location of the compressive pathology, the presence of axial neck pain, the number of segments involved, and the presence of previous surgeries. The following sections discuss the different approaches and various operative techniques described in the treatment of CSM.
Anterior Approach
Indications for an Anterior Approach
The spinal cord can be compressed by herniated discs, spondylotic bars, and uncovertebral osteophytes. Direct decompression of the cord and nerve roots from these degenerative changes can be accomplished with an anterior approach. An anterior approach also allows the surgeon to relieve directly any compression on the anterior spinal artery that has been shown to supply the ventral 75% to 80% of the spinal cord. For most patients with stenosis confined to only one or two levels, an anterior approach provides adequate decompression and is the procedure of choice for most surgeons.43,44 Yonenobu and colleagues44 recommended that an anterior procedure should be confined to the treatment of spondylosis involving no more than three levels, whereas a posterior procedure should be reserved for the treatment of spondylosis involving four levels or more.
The sagittal alignment of the spinal column is an important factor when deciding on an anterior versus a posterior approach. Cervical kyphosis and degenerative instability are clear indications for an anterior approach.37,45 In patients who have lost the normal cervical lordotic curvature or who have developed a kyphotic deformity, a posterior decompression alone may destabilize the cervical spine and may lead to a progression of the deformity. Anterior surgery allows for direct decompression of the neural elements, fusion of the involved segments, and possibly reconstitution of the normal sagittal contours. An anterior approach may also be favored in patients who complain of preoperative neck pain. Two techniques described in anterior decompression and surgical treatment of cervical myelopathy include anterior cervical discectomy and fusion (ACDF) and anterior cervical corpectomy and fusion (ACCF).
Surgical Techniques
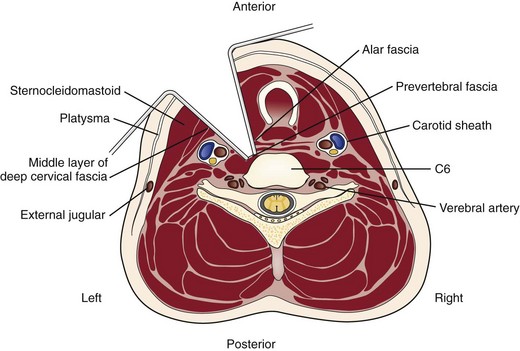
A transverse incision may be used for procedures involving one or two levels; an oblique, longitudinal incision along the course of the medial border of the sternocleidomastoid muscle may be needed for procedures involving three or more vertebral segments. The location of the transverse incision depends on what levels are involved in the surgical procedure (Fig. 41–1). Palpable landmarks of the anterior neck provide guidance as to the appropriate location for this incision. The C1-2 interspace is located at the angle of the mandible, whereas the hyoid bone usually lies anterior to the C3 level. The superior portion of the thyroid cartilage marks the position of the C4-5 interspace. The C6 level can be identified by palpation of the cricoid cartilage or by palpation of the carotid tubercle, which projects anteriorly from the transverse process of the C6 vertebral segment. Care should be taken to orient the transverse incision along the skin creases of the anterior neck to leave a cosmetic-appearing scar.
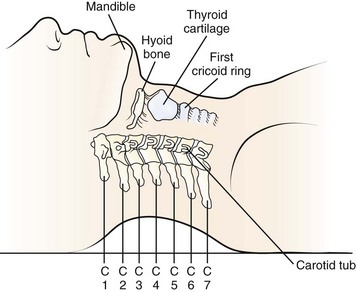
FIGURE 41–1 Palpable bony landmarks and their relationship to cervical spine.
(From Hoppenfeld S: Physical Examination of the Spine and Extremities. Norwalk, CT, Appleton & Lange, 1976, p 110.)
The skin and subcutaneous tissues are incised sharply with a scalpel. The platysma is divided in line with the skin incision with the transverse and longitudinal incisions. Superficial veins, especially the external jugular vein and its branches, must be protected or ligated if they cross the planes of dissection. Next, the medial border of the sternocleidomastoid is identified. Dissection is carried out through the superficial layers of the investing deep cervical fascia between the sternocleidomastoid and the medial visceral muscle column (Fig. 41–2).
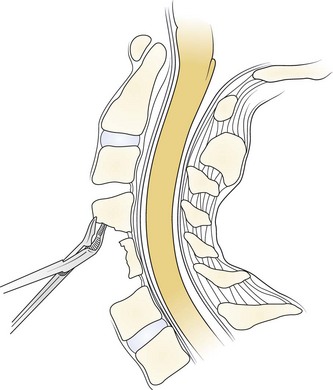
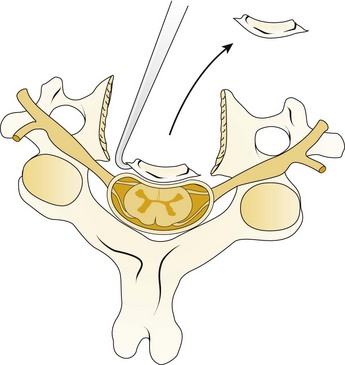
A Leksell rongeur is used to create the most ventral portion of the corpectomy trough (Fig. 41–3). The use of a motorized bur without the creation of this initial trough is dangerous and could result in injury to the carotid vessels or the esophagus if the bur “jumps” off of the anterior vertebral body cortex. After the anterior cortex of the vertebral bodies has been removed, a 5-mm power bur is used to widen and deepen the through. Starting and stopping the bur within the bony trough avoids potential injury to the surrounding soft tissue structures. Resection is continued posteriorly until the posterior cortex of the vertebral bodies is encountered. Bleeding from the cancellous bone can be controlled with bone wax. A smaller bur may be used to thin the posterior cortex and to remove the osteophytes at the uncovertebral joints. The remaining thin shell of the posterior cortex can be removed with small angled curets by pulling the bone away from the posterior longitudinal ligament and the dura (Fig. 41–4).
The posterior longitudinal ligament can be carefully removed with a nerve hook and a small Kerrison rongeur. One should use caution in the presence of ossification of the posterior longitudinal ligament (OPLL) because the ligament may be incorporated into the dura.46 Removal of the ligament should not be attempted in this situation because it may create a dural tear. The cartilage endplates at the superior and inferior extent of the corpectomy are removed with the use of a curet or a bur. A safe and adequate decompression of the spinal cord requires approximately a 15- to 19-mm wide trough.47,48 This trough can be measured directly with a caliper or estimated by comparing the width of the trough with the width of the surgeon’s finger. Foraminotomies may be performed at each of the decompressed levels if the patient’s pathology and symptoms warrant foraminal decompression.
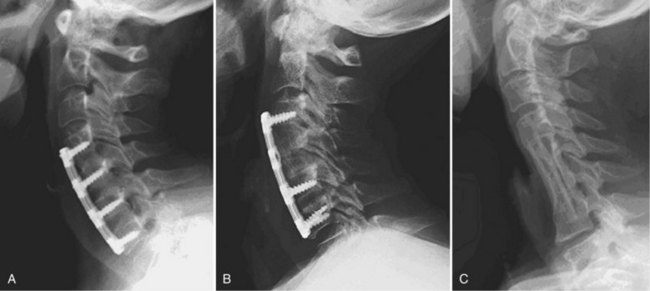
After complete decompression of the neural elements has been accomplished, the endplates are prepared for insertion of the graft. A bone tamp and mallet are used to tap the graft gently in the appropriate position while traction is being applied to the head (Fig. 41–5). Heller1 recommended a maximum of 25 lb of traction. Care must be taken to countersink the graft 1 to 2 mm behind the anterior cortex without forcing it into the canal posteriorly. A small nerve hook can be passed lateral to the graft in the trough to assess the space between the posterior longitudinal ligament and the posterior surface of the graft. The traction is released, and graft stability is assessed by manual flexion and rotation of the head by the anesthesiologist. If an anterior cervical plate is being used, it is placed at this time. The plate helps to prevent anterior migration of the graft and may provide graft compression to improve healing potential.
The wound is closed in layers over a suction drain, which is left in place for 1 to 2 days postoperatively to minimize the risk of postoperative airway compromise secondary to hematoma formation. The patient is placed into a cervical collar for additional immobilization. The choice of collar often depends on surgeon preference, number of levels fused, and presence or absence of instrumentation. After multilevel corpectomies with prolonged retractor times, many surgeons elect to keep the patient intubated overnight to minimize the possibility of respiratory distress from postoperative edema and swelling.49
Anterior Cervical Discectomy and Fusion
The use of anterior cervical decompression and fusion for the treatment of ventral pathology has been consistently reported to be a safe and effective procedure.50,51 Indications for ACDF in the treatment of cervical myelopathy include compression from any disk herniation or spondylotic degeneration that is confined to the disk level.
Although the removal of osteophytes has been reported to improve recovery in patients with CSM, controversy exists regarding the need for an osteophytectomy.52 Robinson and colleagues53 and Connolly and colleagues54 reported complete remodeling and resorption of osteophytes in the presence of a solid fusion. Bohlman22 reported excellent results in 16 of 17 patients who underwent ACDF and who were treated without any osteophyte removal. No patients experienced a loss of function, and all but one of the patients had improvement in functional status. Conversely, Stevens and colleagues55 reviewed CT myelograms of 53 patients who underwent ACDF and reported that at 12 years of follow-up no patients showed any evidence of osteophyte resorption. These authors recommended the systematic removal of all osteophytes to decrease the incidence of persistent postoperative symptoms.
The removal of a thickened ligament or an OPLL may also allow for a more thorough decompression of the spinal cord and may be necessary for the safe exploration and removal of disc fragments that may have become sequestered behind the posterior longitudinal ligament. The surgeon must always be mindful that the removal of the posterior longitudinal ligament increases the risk of developing a cord contusion or a postoperative hematoma.56 In the case of an OPLL, care must be exercised during the removal of the ligament because the dura of the spinal cord may also be ossified.46
Using ACDF in the treatment of 121 patients with CSM, Zhang and colleagues57 observed a 90% improvement in overall neurologic outcome. These authors noted an 85% fusion rate in association with the use of autogenous bone graft, whereas they noted a 50% fusion rate with the use of allograft. They also observed poorer clinical outcomes in patients who developed a pseudarthrosis. With an average number of 3.1 levels fused, Yang and colleagues58 reported 214 patients who underwent ACDF for treatment of CSM. At last follow-up, improved functional status was noted in 90% of patients, despite a pseudarthrosis rate of 37%. With a mean follow-up of 10 years, Irvine and Strachan59 retrospectively evaluated the long-term results of 46 patients who underwent ACDF for treatment of CSM. At last follow-up, 78% of the 46 patients had improved ability to ambulate, whereas 9% of patients experienced progression of their symptoms.
Anterior Cervical Corpectomy and Fusion
Bernard and Whitecloud29 evaluated multilevel cervical corpectomy. All 21 patients in this series had decompression of three or more vertebral levels with autograft. At an average of 32 months’ follow-up, these authors noted that 76% of patients had an improvement in functional outcome, and no patients developed pseudarthrosis. One patient in this series experienced graft dislodgment. In a study of 27 patients with CSM treated with multilevel corpectomy and fusion, Jamjoom and colleagues60 noted a 96% fusion rate with clinical improvement in 80% to 88% of patients. Although no patients experienced postoperative neurologic deterioration, three patients developed dislodgment of the strut graft.
Emery and colleagues50 reviewed their series of patients with CSM treated with different forms of anterior decompression and fusion procedures without the use of instrumentation. Of 108 patients, 58 were treated with ACCF using either fibular or iliac crest autograft without plate fixation. Six graft-related complications occurred in the corpectomy group, four of which occurred in patients who had been previously treated with cervical laminectomy. Of the patients who underwent corpectomy, a nonunion rate of 5% was observed, and the average improvement in Nurick grade ranged from 2.4 preoperatively to 1.2 at final follow-up.
Zdeblick and Bohlman61 reviewed their results of 14 patients with myelopathy and associated cervical kyphotic malalignment who were treated with anterior corpectomy and fusion using autograft without any plate fixation. The patients were stabilized postoperatively in a halo vest. At follow-up, 12 of the 14 patients had a solid arthrodesis. Nine patients had complete neurologic recovery, and only one patient failed to show any neurologic improvement. Correction of the kyphosis averaged 32 degrees. Zdeblick and Bohlman61 concluded that myelopathy in the setting of cervical kyphosis can be effectively and safely treated with multilevel corpectomy and strut graft reconstruction without instrumentation.
In 1998, Fessler and colleagues62 evaluated the outcomes of 93 patients with CSM who were treated with anterior corpectomy and fusion. Multisegmental involvement was noted in 31 of these patients. Fessler and colleagues62 reported that 86% of patients showed improvement in neurologic scores as measured by Nurick grade. In a more recent study published in 2005, Chibbaro and colleagues63 documented their experience in management of CSM with anterior cervical corpectomy. Using autograft and plate fixation, 54 patients received a one-level corpectomy, 11 patients received a two-level corpectomy, and 5 patients received a three-level corpectomy. At 16 weeks’ follow-up, the authors observed no evidence of pseudarthrosis, and they documented a 94% improvement in functional status. Using the modified JOA scale, no patients experienced any decline in neurologic function compared with their preoperative status.
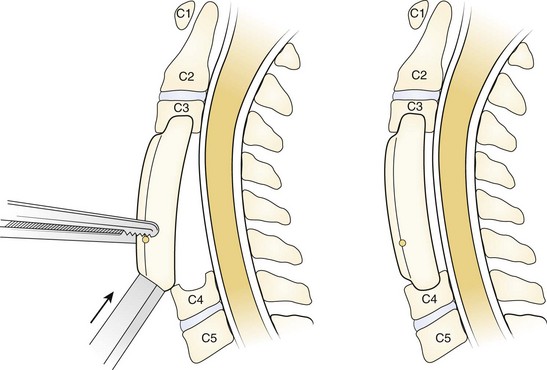
The combination of corpectomy and adjacent discectomy has also been performed as a technique that can be used in treatment of CSM (Fig. 41–6). This method has been described as a hybrid decompression. It is indicated when spinal cord compression is present at a disc space and a vertebral body at different levels. Ashkenazi and colleagues64 published their series of 25 patients who underwent a hybrid decompression with anterior plate instrumentation for the treatment of multilevel CSM; 12 patients underwent a one-level corpectomy and adjacent one-level discectomy, and 13 patients underwent a two-level corpectomy with preservation of an intervening vertebral body. The investigators observed a 96% fusion rate, and 24 of the 25 patients reported either a neurologic improvement or an unchanged status.
Complications
Although an anterior cervical approach has been shown to be a safe and effective procedure in treating patients with myelopathy, suboptimal outcomes have been reported when this approach is used to treat three or more levels.65–67 As more levels are involved in the attempted fusion, increased rates of nonunion have been noted in ACDF and ACCF procedures. Numerous studies have shown an inverse relationship between the fusion rate and the number of vertebral segments involved.68–71 Nonunion is determined by the absence of bridging bone across the graft-host interface on static radiographs and by the presence of motion on dynamic films.
The arthrodesis rate after ACDF without instrumentation was compared in a series by Bohlman and colleagues.72 They reported a fusion rate of 89% of 62 patients after a one-level fusion, 73% of 42 patients after a two-level fusion, and 73% of 11 patients after a three-level fusion. An unsuccessful four-level ACDF was performed in one patient in their series. Bohlman and colleagues72 attributed the lower fusion rate to increased number of fusion interfaces and increased motion as more levels are involved. Emery and colleagues50 reviewed their series of patients with CSM treated with different forms of anterior decompression and fusion procedures without the use of instrumentation. Of 108 patients, 45 were treated with anterior discectomy and fusion; the investigators reported 13 patients in the discectomy and fusion cohort who developed a nonunion. Similar to the observations made by Bohlman and colleagues,72 Emery and colleagues50 observed increased rates of nonunion as the numbers of fused levels increased. Better clinical outcomes were noted in patients who went on to a solid fusion.
Vaccaro and colleagues66 reported a series of patients who underwent an instrumented anterior corpectomy and fusion using a strut graft. They observed a 9% nonunion rate in two-level corpectomies, which increased to a 50% nonunion rate in three-level corpectomies. Sasso and colleagues73 noted a similar trend in increasing nonunion rates in their series of patients who underwent ACCF. They reported a 6% failure rate in two-level corpectomies that increased to a 71% failure rate when three levels were involved. When compared with multiple discectomy and fusion, Hilibrand and colleagues74 observed better fusion rates in patients treated with corpectomy and strut grafting. In their series, all reconstructions were performed with autograft and without anterior cervical plates. A 93% fusion rate was observed in the corpectomy cohort, whereas only a 66% fusion rate was noted in the ACDF cohort.
Although it is a more technically demanding surgery, corpectomy with fusion relies on an arthrodesis to occur at only two interfaces. Because a fusion is required between only two levels, the chance of developing a pseudarthrosis is decreased.61 Swank and colleagues75 reported 38 patients who were treated with multilevel ACDF and 26 patients who were treated with subtotal corpectomy. Allograft and anterior plate fixation were used in all patients. Among patients with two-level disease, the patients treated with two-level discectomy had a 64% fusion rate, whereas the patients treated with one-level corpectomy had a 90% fusion rate. Similarly, among patients with three-level disease, the patients treated with three-level ACDF had a fusion rate of 46%, whereas the patients treated with two-level corpectomy had a fusion rate of 56%.
In noninstrumented ACCF, immediate stability of the graft depends on the graft-host bone interface. The sculpting of the graft and the vertebral endplates and the impaction of the graft into place while the head is in traction contribute to the initial stability of the graft.29,76–78 Graft migration most commonly occurs at the inferior end of the construct. Wang and colleagues79 reported their findings in a retrospective review of 249 patients who underwent corpectomy and fusion with the use of autograft and no plate fixation over a 25-year period. Graft migration was observed in 16 of 249 patients. None of the 16 patients experienced any respiratory or neurologic complications as a result of the graft displacement. Wang and colleagues79 noted an increased rate of this complication occurring in patients undergoing longer fusions and in patients whose fusions ended at the C7 vertebral body.
To increase immediate postoperative stability, anterior plate instrumentation may be added. The addition of anterior plate fixation has been shown to improve the rate of fusion, reduce postoperative immobilization, reduce the incidence of segmental kyphosis, and reduce the prevalence of graft-related complications.54,80,81 The plate may act as a buttress to block graft migration physically.82–84 If plate fixation is performed, it is recommended that a minimum distance of 5 mm be maintained between the plate and the unaffected disc segment to minimize the potential of developing adjacent level disc ossification.85
The use of anterior instrumentation does not absolutely prevent graft complications. Sasso and colleagues73 noted that catastrophic construct failure occurred in five of seven patients who underwent a three-level corpectomy with autogenous iliac crest bone graft and anterior cervical locked plating. Most failures occurred with the graft cavitating into the vertebral body at the inferior end of the construct, which caused the graft to displace anteriorly. The biomechanical effects of long anterior cervical plates after three-level corpectomy and strut graft reconstruction were evaluated in two studies.86,87 The authors of the studies believed that the failures typically seen with instrumented multilevel corpectomies treated with long anterior cervical plates were the result of pistoning of the inferior aspect of the graft. Vaccaro and colleagues66 noted a 50% failure rate of the patients in their series who underwent three-level ACCF with anterior plate fixation. Based on their findings, they recommended the addition of a posterior stabilizing procedure to supplement multilevel ACCF.
Delayed fractures have been reported through fibular strut grafts after multilevel ACCF.88,89 Some authors support the use of a titanium cage to achieve immediate construct rigidity.90,91 The use of this construct may avoid the potential complication of late strut graft fracture. These cages are packed with bone and can be used in combination with anterior instrumentation after ACCF. Excellent fusion rates have been reported using this technique; however, caution must be exercised with the use of this construct in osteoporotic bone.90,91
Although graft complications have been reported extensively in the literature, they are not the only problem. Injury to the vertebral arteries has also been reported during ACCF procedures.61,74 In patients who underwent corpectomy, Eleraky and colleagues92 reported a prevalence of vertebral artery injury of 2% in 185 patients. It is imperative that a surgeon maintain a midline orientation during an anterior decompression to avoid any violation of the lateral wall and subsequent injury to the vertebral artery. This orientation is particularly important in patients who have tortuosity of the vertebral artery.93 Anatomic landmarks that have been discussed include the medial margin of the uncovertebral joint, medial margin of the longus colli muscle, and natural curve of the vertebral endplate. Studies have shown that by leaving a margin of approximately 5 mm to the medial border of the foramen transversarium, a total central decompression of approximately 15 mm at C3 and 19 mm at C6 can be performed safely.47,48
Individual patient characteristics or comorbidities may influence the surgeon’s decision to supplement an anterior decompression with allograft or autograft. Traditionally, the use of iliac crest autograft has been the “gold standard” in one-level and two-level anterior decompression and fusion procedures. For longer fusions, most surgeons prefer to use a structural fibula strut graft. The morbidity associated with the graft harvest is associated with an increased complication rate. Specific iliac crest donor site complications include neuroma formation, iliac crest fracture, cosmetic deformity, persistent pain, and infection.94–96 In addition to infection, harvesting autogenous fibula has been associated with injury to the peroneal nerve, contracture of the flexor hallucis longus and flexor digitorum longus tendons, development of lower extremity deep venous thrombosis, stress fractures of the tibia, and chronic ankle pain.97–100
To avoid the complications associated with the harvest of autograft, some authors advocate the use of allograft. The use of allograft in multilevel fusion has historically been associated with higher rates of nonunion.101 In a retrospective study of 126 multilevel discectomy and corpectomy cases, Fernyhough and colleagues102 compared the fusion rates between fibula strut autograft and fibula strut allograft. They noted a 27% nonunion rate in the autograft group versus a 41% nonunion rate in the allograft group. Conversely, MacDonald and colleagues103 reported a 97% fusion rate in their series of patients who underwent a decompressive corpectomy with the use of fibula strut allograft. More recently, Samartzis and colleagues104 published a series that showed equivalent rates of fusion between allograft and autograft in patients who underwent multilevel ACDF using cervical plates and current surgical techniques.
Adjacent segment degeneration in previously fused segments in the cervical spine has been reported in the literature. Based on a long-term follow-up study of 374 patients who underwent anterior cervical fusion, Hilibrand and colleagues105 reported a 25% risk of development of adjacent segment disease within 10 years. In their published series, these authors noted that the C5-6 and C6-7 levels were the most frequently involved. They reported that the risk of developing adjacent segment disease was more likely a manifestation of the natural aging process rather than a consequence of the fusion. More recently, Rao and colleagues106 reported that performing ACDF with plate fixation does not lead to the development of adjacent segment disease.
Postoperative radiculopathy is a well-recognized phenomenon that occurs after posterior decompression of the spinal cord.23,107,108 This complication has also been reported in the treatment of CSM in anterior decompressive procedures. Saunders and colleagues65 reported an incidence of C5 palsy of 20% in their series of 96 patients who underwent corpectomy to treat CSM. They recommended limiting the ventral trough to 14 or 15 mm. Yonenobu and colleagues108 reported a prevalence of postoperative radiculopathy of 3.9% of 204 patients after anterior procedures. A proposed etiology for the development of the palsy is secondary to an impingement of the ventral aspect of the spinal cord against the edges of the corpectomy trough. The removal of osteophytes can increase the risk of inadvertent injury to the spinal cord itself. Yonenobu and colleagues109 reported a single patient in their series of 75 patients who sustained an injury to the cord after the resection of posterior osteophytes during ACDF. Subsequently, these authors recommended that corpectomy be considered in patients with posterior osteophytes that are substantial enough to require removal.
Injuries to the soft tissue structures can occur during an anterior approach to the cervical spine. Swallowing difficulties are the most common postoperative problems encountered. Although a frequent complaint, the dysphagia most commonly follows a transient course.110 This problem seems to be related to esophageal dysmotility that can result from excessive retraction. Bazaz and colleagues110 reported a 50% prevalence of dysphagia at 1-month follow-up that was more frequently noted when multiple levels were involved in the fusion. At 1-year follow-up, of the 197 patients involved in this study, only 12.5% continued to complain of symptoms. Instrumentation has not been shown to increase the risk of dysphagia.110,111 Esophageal injury can occur secondary to excessive retraction, electrocautery, or perforation from sharp instruments. The incidence of esophageal perforation has been reported by Newhouse and colleagues112 to be approximately 0.25%.
Injuries to the recurrent laryngeal nerve have been reported among complications related to the dissection and mobilization of soft tissues in the neck. In a series of 650 patients who underwent an anterior cervical procedure, Frempong-Boadu and colleagues113 reported a 2% prevalence of injury to the recurrent laryngeal nerve. In contrast, Yue and colleagues114 reported a prevalence of recurrent laryngeal nerve injury of 11% in 85 patients. Injury to the laryngeal nerve is most commonly attributable to compression within the endolarynx.115 Apfelbaum and colleagues115 observed a significant decrease in the occurrence rate of this complication from 6.8% to 1.7% by deflating and reinflating the endotracheal cuff after the retractors were placed. After a review of the literature, Baron and colleagues116 reported an overall incidence of 4.9% for hoarseness and 1.4% for unilateral vocal cord paralysis. Other causes of nerve injury include direct trauma or an indirect pressure or stretch injury induced by a hematoma or by prolonged retractor placement.
Beutler and colleagues117 compared the rate of injury to the recurrent laryngeal nerve during right-sided and left-sided approaches. Although they found no difference in the rate of postoperative dysphonia between the two sides, they did observe a generally higher complication rate in the setting of revision surgery. For this reason, some surgeons advocate approaching the spine from the opposite side in a revision setting to avoid the frustration and dangers of operating through scar tissue. If a revision surgery is contemplated, a formal evaluation of vocal cord function must be done if the surgeon intends to approach the spine from the opposite side. In a situation in which the original surgery resulted in permanent unilateral vocal cord paralysis, an opposite-sided approach would be inadvisable because the potential for bilateral vocal cord paralysis would be catastrophic.
Prolonged retraction can also cause injury to the cervical sympathetic ganglion resulting in Horner syndrome, characterized by ipsilateral ptosis, miosis, and anhidrosis. This complication can also be seen in revision surgeries or in operations involving the cervicothoracic junction. Bertalanffy and Eggert56 reported an incidence of 1.1% in their series of 450 patients who underwent an anterior cervical fusion. To prevent this complication, the longus colli muscles should be dissected of the anterior vertebral body in a subperiosteal fashion, and retractors should be placed deep to the longus colli muscles.
Posterior Approach
Indications for a Posterior Approach
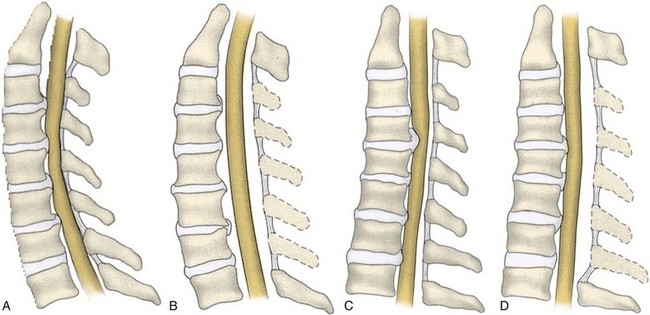
Before the 1950s, the operative treatment of degenerative cervical disorders primarily occurred through a posterior approach. The posterior approach to the cervical spine has continued to be a safe and effective treatment option in the surgical management of CSM. Indications for a posterior approach to the cervical spine include congenital cervical stenosis, multilevel cervical spondylosis, OPLL, ossification of the yellow ligament, and posterior compression caused by infolding of the ligamentum flavum.37,45,118,119 A posterior approach to the cervical spine relieves spinal cord compression through two distinct mechanisms: direct and indirect decompression. When the pathology causing the myelopathy is primarily due to ventral structures, posterior procedures are indirect methods of decompression. With indirect decompression, expansion of the canal allows the spinal cord to shift posteriorly away from the anterior impinging structures. In situations in which the myelopathy is secondary to a congenital stenosis or a redundant ligamentum flavum, the decompressive effect of a posterior procedure is more direct. The removal or relocation of the posterior impinging structures acts to decompress the spinal cord and is a form of direct decompression. Laminectomy and laminoplasty are two techniques of decompressing the cervical spine from a posterior approach.
For an effective indirect spinal cord decompression to occur, certain prerequisites must exist. Posterior procedures are primarily indicated in the presence of a neutral or lordotic sagittal alignment to allow for the posterior translation of the spinal cord.120 As spondylosis progresses, there is a general trend toward loss of lordosis.121 In a patient with a kyphotic cervical spine, the sagittal alignment of the bony elements does not allow for the posterior translation of the spinal cord (Fig. 41–7). Even after a posterior decompression, the cord remains draped over the anterior compressive pathology. A minimal lordotic curvature of 10 degrees should be present if posterior decompression is considered.122 Studies have shown that measured from C2 to C7, the average lordotic curvature in a normal cervical spine is 14.4 degrees.123
Yamazaki and colleagues124 observed continued ventral compression after posterior decompression in patients with anterior canal encroachment greater than 7 mm and with lordotic sagittal alignments of less than 10 degrees. When a posterior decompression is performed in patients with a kyphotic cervical alignment, modest improvements in neurologic outcome can be expected. The recovery achieved from a decompression in the presence of a cervical kyphosis tends to be inferior, however, when compared with a posterior decompression that is performed in the presence of a cervical lordosis.37,125 These findings were echoed by Satomi and colleagues,125 who found that patients with maintenance of preoperative lordosis exhibited a superior improvement in JOA score compared with patients in whom the lordosis decreased by 10 degrees or more.
In patients who underwent decompressive laminoplasty, Sodeyama and colleagues45 showed that patients with lordotic spines had the greatest postoperative posterior translation of the spinal cord with an average shift of 3.1 mm. Patients who had a neutral alignment showed a peak posterior shift of less than 3 mm, and patients who had a kyphotic cervical alignment showed a peak posterior shift of less than 2 mm. In a study evaluating 114 patients with CSM after laminoplasty, Suda and colleagues37 observed that a preoperative local kyphosis exceeding 13 degrees provided patients with the worst prognosis for neurologic recovery. They concluded that cervical kyphosis should be regarded as a relative contraindication for a posterior decompression.
As a second prerequisite, indirect spinal cord decompression also requires a sufficient length of canal expansion. Decompression of the posterior structures at levels beyond areas of focal stenosis may allow for greater translation of the cord. The degree of canal expansion that is achieved via a posterior approach has been shown to correlate with the success of postoperative recovery.126–128 A statistically significant difference in recovery rate was reported by Hirabayashi and colleagues126 when the anteroposterior diameter of the spinal canal increased greater than 5 mm versus an increase of less than 2 mm. Ishida and colleagues127 observed better recovery rates in patients in whom posterior decompression increased the sagittal diameter of the canal to 15 mm or greater. Kohno and colleagues128 showed that expansion of the anteroposterior canal diameter to 12.8 mm correlated with a good postoperative recovery after laminoplasty. Shaffrey and colleagues129 reported an overall average increase in the spinal canal cross-sectional area of 55% after posterior decompression with an 88% increase at the most compressed level.
When adequately decompressed, the spinal cord has been reported to change in morphology from a flattened to a more natural oval shape. In a CT myelography study, Aita and colleagues120 described changes in spinal cord morphology in 38 patients after open-door laminoplasty. Postoperatively, the largest increase in mean spinal cord cross-sectional area was improved by 12%. In addition, the mean sagittal cord diameter was enlarged by 1 mm, and the mean coronal diameter was decreased by 1 mm.
The longitudinal height of the cervical spine as a prognostic indicator for neurologic recovery has also been evaluated. Chiba and colleagues130 noted that after extensive open-door laminoplasty, patients with CSM were more likely to have improved neurologic results compared with patients with OPLL. They observed a greater degree of preoperative cervical spine shortening owing to disc degeneration in patients with CSM. These authors attributed the improved recovery rate in these patients to the redundancy within the spinal cord after decompression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree