CHAPTER 43 Cervical Disc Replacement
Background
Anterior cervical discectomy and fusion (ACDF) is a proven intervention for patients with radiculopathy and myelopathy.1 It has served as the standard by which other cervical and spinal disorders may be judged as the result of its high rate of success. The success of this technique is often judged based on its consistent ability to relieve symptoms related to neurologic dysfunction. In this sense, the clinical results with regard to the patient’s index complaint are outstanding. The radiographic results of this technique are also initially predictable with a high rate of fusion. Plating techniques have diminished the need for postoperative immobilization or eliminated them entirely.2 Because of limitations specific to this procedure, investigators have developed surgical alternatives to fusion that attempt to address the kinematic and biomechanical issues inherent in it.
A major concern related to the treatment of cervical degenerative disc disease and spondylosis with ACDF is the issue of adjacent segment degeneration. This event is manifest as the radiographic appearance of degenerative change at a level directly above or below a level treated with a surgical intervention—typically being associated with degeneration of a level adjacent to a fused level. The incidence of this phenomenon has been reported to be 92% by Goffin and colleagues,3 who wrote a long-term follow-up on patients after treatment with anterior interbody fusion.
Although some debate remains regarding the causation of adjacent segment degeneration—with a mix of postsurgical and naturally determined aging cited as root causes—there is little debate regarding the existence of this phenomenon. It is also relevant to note the clinical distinction between adjacent segment degeneration and adjacent segment disease. Adjacent segment disease is defined as adjacent segment degeneration that causes clinical symptoms (pain or neurologic disorders or both) severe enough to lead to patient complaint or require operative intervention.4 Although this distinction has not remained consistent in published literature, it is an important consideration with regard to the phenomenon that occurs in discs adjacent to discs that have undergone a surgical intervention. Numerous studies have made a consistent point of distinguishing between radiographic degeneration and symptomatic disease.3,5
There is clinical evidence to support the postsurgical nature of adjacent segment disease. In patients previously treated with fusion, adjacent segment disease has been documented at a rate of 2.9% of patients per annum by Hilibrand and colleagues,4 and 25% of patients undergoing cervical fusion have new onset of symptoms within 10 years of fusion. This study has received a great deal of attention and has led to further investigations into biomechanical causation. Other reports have focused on the recurrence of neurologic symptoms and degenerative changes adjacent to fused cervical levels.3,6 The concept that adjacent levels need to compensate for loss of motion in the fused segment may also be valid. Segments adjacent to a fusion have an increased range of motion and increased intradiscal pressures.7,8
Bone graft materials used in traditional ACDF procedures have also been a source of controversy. Current ACDF techniques make use of allograft bone, premanufactured allograft bone, and autologous iliac crest. Complications associated with autologous iliac crest harvest used as a fusion graft in ACDF are well documented. Sandhu and colleagues9 reported a complication rate of 1% to 25% with such procedures. Complications such as acute and chronic pain, infection, meralgia paresthetica, and pelvic fracture are known to occur at harvest donor sites.10,11
Although allograft removes the risks associated with the harvest of autograft, it has the detriment of having the theoretical risk of disease transmission. In practice, this risk is believed to be extremely minute, although the U.S. Food and Drug Administration (FDA) has taken this issue seriously. The issues of disease transmission and contaminated graft materials have been highlighted by allograft tissue recalls by the FDA in recent years.12 Although bone graft substitutes may play a role in the future practice of ACDF, this continues to be a minority stake in the overall graft selection of modern surgeons.
Pseudarthrosis is another complication encountered with anterior cervical fusion procedures. Pseudarthrosis is the failure of bony bridging or nonunion of a segment that has previously been treated with a bone graft or a bone graft substitute—an attempt at fusion has been made. In multilevel ACDF procedures, there is a relationship between the rate of pseudarthrosis and the number of levels fused. Brodke and Zdeblick13 reported a 97% fusion rate in single-level ACDF, which decreased to 83% with fusion at three levels. Bohlman and colleagues1 reported an 11% pseudarthrosis rate in single-level fusions that increased to 27% with multilevel fusions.
In recent years, the use of bone morphogenetic proteins has been proposed as an alternative or adjunct to traditional bone grafting techniques to combat the pseudarthrosis issue in patients deemed to be at higher risk for this complication.14,15 This off-label use has been associated with an increased incidence of swelling complications and concerns for graft resorption and migration of interbody implants.16–19 These issues serve to strengthen the argument for fusion alternatives in the treatment of discogenic pathology in the anterior cervical spine.
Total intervertebral disc replacement (TDR) is designed to preserve motion, avoid limitations of fusion, and allow patients to return quickly to routine activities. The primary goals of the procedure in the cervical spine are to restore disc height and segmental motion after removing local pathology that is deemed to be the source of a patient’s index complaint. A secondary intention is to preserve normal motion at adjacent cervical levels, which may be theorized to prevent later adjacent level degeneration. Cervical TDR avoids the morbidity of bone graft harvest.20,21 It also avoids complications such as pseudarthrosis, issues caused by anterior cervical plating, and cervical immobilization side effects.
History of Disc Arthroplasty and Device Design
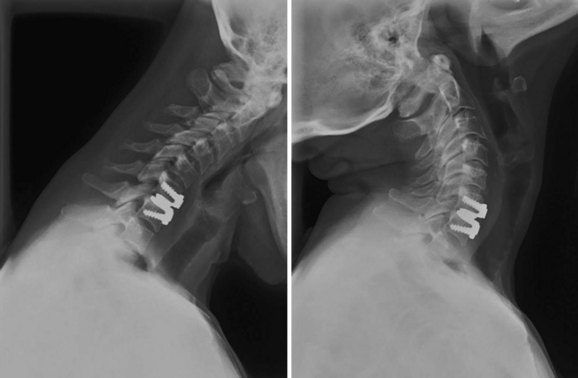
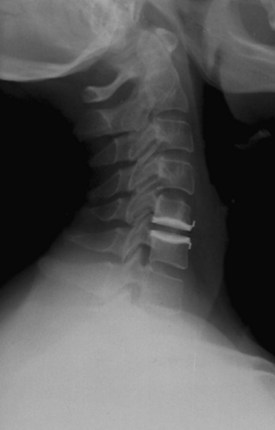
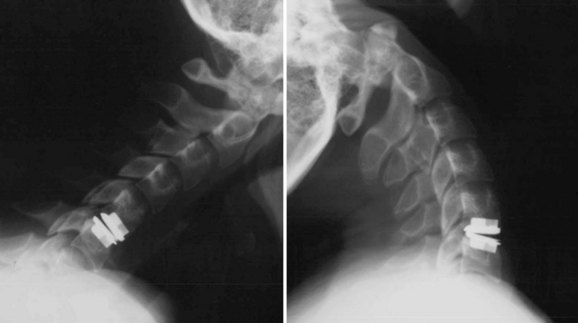
An understanding of the evolution of cervical TDR serves as an important lesson in the concepts of device design, TDR bearing and wear characteristics, and articular constraint. In the late 1980s, Cummins and colleagues22 developed a metal-on-metal ball-and-socket cervical disc replacement composed of 316L stainless steel. With the acquisition of this technology and the later development of new metal-on-metal devices came a rapid transition from this device to the most recent device, the PRESTIGE LP (Medtronic Sofamor Danek, Memphis, TN). A predecessor of this device, the PRESTIGE ST (Medtronic Sofamor Danek, Memphis, TN) is currently approved by the FDA for human use in the United States (Figs. 43-1 and 43-2). More recent additions to the metal-on-metal category of arthroplasty devices include the Kineflex-C disc (Spinal Motion, Mountain View, CA) and the CerviCore intervertebral disc (Stryker Spine, Allendale, NJ) (Figs. 43-3 and 43-4), which are in the process of U.S. FDA investigational device exemption (IDE) trials.
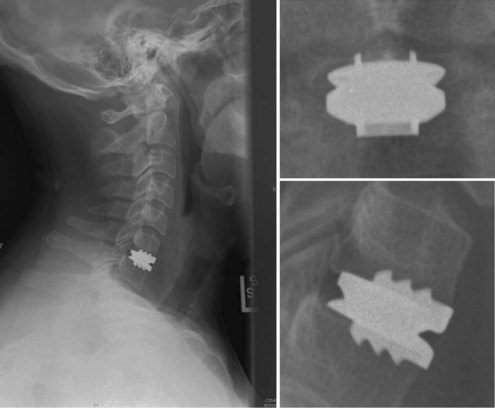
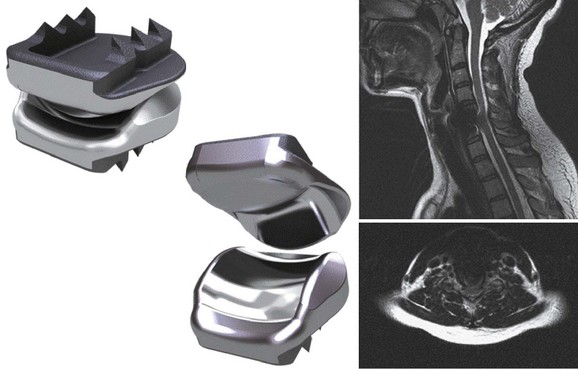
Numerous devices have evolved in parallel to the metal-on-metal implants, including the BRYAN cervical disc (Medtronic Sofamor Danek, Memphis, TN) (Figs. 43-5 through 43-7), the PCM (CerviTech, Rockaway, NJ), the DISCOVER (DePuy Spine, Raynham, MA), and the MOBI-C (LDR, Austin, TX). Each of these devices is in the process of limited human trials or U.S. FDA IDE submission and represents an alternative to metal-on-metal bearing surfaces, which have the potential for metal debris and systemic concentration of metal ions. To date, one such device, the Prodisc-C (Synthes Spine, Paoli, PA) (Figs. 43-8 and 43-9), has obtained approval for use in the United States.
A summary of the design characteristics of each of these devices is presented in Table 43–1. Although the ideas of bearing surface, wear debris, and constraint are not new to discussions with regard to arthroplasty in general, they are relatively new in regard to the spine. A full understanding of the term constraint has not been agreed on because constraint may arise within the device or as a result of the local anatomy (e.g., facets, posterior longitudinal ligament). As the knowledge base in spine TDR increases, intelligent investigations and discussions are sure to include many of these concepts and may redefine understanding of them.
Indications for Use, Contraindications, and Complications
Cervical disc arthroplasty trials have included patients refractory to nonoperative treatment modalities with and without radiculopathy or myelopathy or both and with one-level and two-level degenerative disc disease or spondylosis.23–25 These indications have been retained throughout the FDA approval process. At the time of this writing, two devices, the PRESTIGE ST and the Prodisc-C, have achieved FDA approval for single-level use in the United States. Other devices are in various stages of the IDE and approval process (Table 43–2). ACDF may be discussed as part of the indication process for an arthroplasty procedure. The historical challenges associated with ACDF presented in this chapter may be weighed against the early nature of data with respect to cervical arthroplasty in a patient’s informed consent discussion.
TABLE 43–2 Current Status of Cervical Arthroplasty Devices in the United States*
| Device | Manufacturer | U.S. FDA Status |
|---|---|---|
| PRESTIGE-ST | Medtronic Sofamor Danek | Approved |
| Prodisc-C | Synthes Spine | Approved |
| BRYAN disc | Medtronic Sofamor Danek | IDE data submitted, approval pending |
| CerviCore disc | Stryker Spine | IDE in progress |
| DISCOVER disc | DePuy Spine | IDE in progress |
| Kineflex-C disc | Spinal Motion | IDE in progress |
| MOBI-C disc | LDR | IDE in progress |
| PCM disc | Cervitech | IDE in progress |
| PRESTIGE-LP | Medtronic Sofamor Danek | IDE in progress |
| SECURE-C | Globus Medical | IDE in progress |
IDE, investigational device exemption.
* Table current as of April 1, 2009.
The appropriate time to discuss complications related to cervical arthroplasty is at the time of informed consent. The approach-related risks of cervical arthroplasty are similar to ACDF and should be discussed as such. These risks have been adequately covered in other portions of this text. A unique risk of arthroplasty is the concern of heterotopic ossification in the region of the arthroplasty device.26–30 Heterotopic ossification may result in loss of motion or frank fusion of the index level. Heterotopic ossification may be associated with the physiologic response to the implantation process, the amount of bleeding or hemostasis necessary in a particular procedure, or the amount of bone debris created at the time of preparation for implantation. Postoperative use of nonsteroidal anti-inflammatory drugs has been suggested to moderate the prevalence of this risk.
Other complications may occur with cervical arthroplasty that are independent of the anterior cervical approach, including infection, implant migration, subsidence, continued or new neurologic findings, vascular injury, dural injury or cerebrospinal fluid leak, hematoma, and reoperation for adjacent level disease. Many complications associated with placement of arthroplasty devices have come to light as the result of reporting and analysis of the prospective randomized multicenter U.S. FDA IDEs and large studies performed outside the United States.23–25,31,32
Technique of Implantation
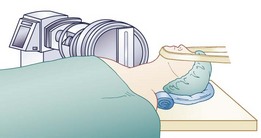
Intraoperatively, patient position is important. A “physiologic” or slightly lordotic cervical spine position is preferred.33 Assessment via fluoroscopy (C-arm) is crucial to patient positioning and implant insertion and fixation portions of these procedures. It is important to keep the head, neck, and shoulders in a stable and neutral position throughout this surgical procedure. A small towel roll may be placed under the neck to assist with appropriate positioning of the neck and shoulders and to keep a physiologic lordosis without creating a hyperlordosis (Fig. 43–10). This positioning technique differs from the typical placement of a roll under the shoulders or thoracic spine, which could place the cervical spine in hyperlordosis. The head is placed on a doughnut-type pillow or a folded towel to keep it from rolling during the procedure. The careful positioning of shoulders with a taping technique can also allow for less motion during this procedure and must be carefully weighed against the risk of traction to the shoulders. Taping of the shoulders differs from the commonly used wrist restraints in a typical ACDF procedure that are used to obtain additional longitudinal traction via a temporary “pull” on the arms.
After neurologic decompression, assessment for placement of an appropriately sized disc and planning for proper orientation of the implant are crucial to successful arthroplasty. To this end, it should be the surgeon’s goal to place as large a device (with respect to diameter) as possible in the prepared space.35 Device-specific tools may aid in this assessment.
Postoperative Imaging
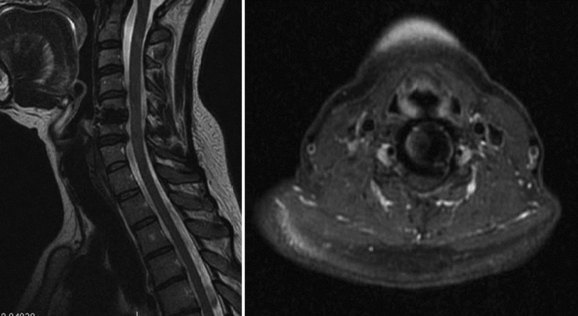
To moderate the risks of technique and radiation associated with CT myelography, MRI has been proposed as an alternative that allows for postoperative assessment of neurologic status adjacent to and at the level of a prior cervical arthroplasty procedure. Success with this technique has been described by Sekhon and colleagues34 in several devices, including the BRYAN and the PRESTIGE LP arthroplasty devices. Both of these devices use titanium alloy in their endplates, which was shown to produce less MRI artifact at the index and adjacent surgical levels than the metals associated with the manufacture of the Prodisc-C and PCM arthroplasty devices.34 The BRYAN prosthesis has a polyurethane core, and the PRESTIGE LP had a titanium carbide alloy (as tested in the study). Investigators had difficulty assessing the neural structures at the index and adjacent levels with devices manufactured with nontitanium metals (cobalt-chromium-molybdenum) used in the Prodisc-C and PCM devices.