Bone–forming (osteogenic) lesions
Osteoid osteoma, osteoblastoma, osteosarcoma
Cartilage–forming (chondrogenic) lesions
Osteochondroma, enchondroma, chondroblastoma, chondromyxoid fibroma, chondrosarcoma
Fibrogenic, fibro–osseous fibrohistiocytic lesions
Nonossifying fibroma, fibrous dysplasia, osteofibrous dysplasia, fibrosarcoma, malign fibrous histiocytoma
Round cell lesions
Langerhans cell histiocytosis, Ewing sarcoma-PNET, lymphoma, myeloma
Other/tumor–like lesions
Simple bone cyst, aneurysmal bone cyst, giant cell tumor
Table 35.2
Classification of bone tumors based on their biological behavior
Benign lesions |
Osteoid osteoma, osteochondroma, enchondroma, nonossifying fibroma, fibrous dysplasia, simple bone cyst, Langerhans cell histiocytosis |
(Benign but) locally aggressive lesions |
Osteoblastoma, chondroblastoma, osteofibrous dysplasia, chondromyxoid fibroma, aneurysmal bone cyst, giant cell tumor |
Malignant lesions |
Osteosarcoma, chondrosarcoma, malign fibrous histiocytoma, Ewing sarcoma-PNET, multiple myeloma-plasmacytoma |
Table 35.3
Age ranges of frequent bone tumors
Benign | Malignant | |
|---|---|---|
0–5 years | Langerhans cell histiocytosis | Ewing sarcoma |
5–10 years | Simple bone cyst | Osteosarcoma |
Aneurysmal bone cyst | Ewing sarcoma | |
Nonossifying fibroma | ||
Fibrous dysplasia | ||
Osteoid osteoma | ||
Langerhans cell histiocytosis | ||
10–20 years | Fibrous dysplasia | Osteosarcoma |
Osteoid osteoma | Ewing sarcoma | |
Aneurysmal bone cyst | ||
Chondroblastoma | ||
Fibrous dysplasia | ||
Adult | Giant cell tumor | Multiple myeloma |
Enchondroma chondrosarcoma | ||
Metastatic carcinoma |
Plain radiographs give the most detailed information about a skeletal lesion. At least two views should be obtained. Radiographic changes may remain undetected until 30–40 % of cortical destruction is reached. Plain radiographs have a limited role in exhibiting soft tissue extension of skeletal tumors. Soft tissue extension and intramedullary involvement are best viewed by magnetic resonance imaging (MRI) [1]. MRI is an essential imaging modality in staging, assessing response to neoadjuvant chemotherapy and evaluation of long-term follow-up of the skeletal malignancies. MRI of the whole affected bone is important to detect skip metastases. Epiphyseal and neurovascular involvement should also be carefully observed. Post-neoadjuvant chemotherapy MRI should be well evaluated; change in size and peripheral edema of the tumor should be compared with initial MRI.
Computerized tomography (CT) is beneficial in axial skeleton involvement due to its complex anatomy. CT is also preferred when cortical integrity and intralesional mineralization is questioned. A whole body bone scan indicates primary lesion’s biological activity as well as accompanying skeletal lesions.
Radiographic Properties of Skeletal Lesions
Localization and Origin
Epiphyseal, metaphyseal, and diaphyseal
Medullary (central, eccentric), intra-cortical
Patterns of Bone Destruction (Fig. 35.1)
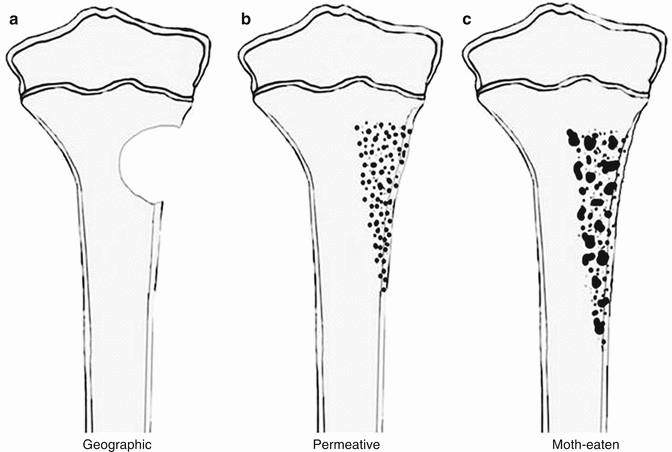
Fig. 35.1
Type of bone destruction. (a) geographic, (b) permeative, (c) moth-eaten destruction
Geographic destruction; benign, slowly growing lesions
Permeative, moth-eaten destruction; aggressive, rapidly growing lesions [2].
Response of the (Host) Bone
Limited within intact cortices, static lesions
Impaired cortical integrity and soft tissue extension, aggressive lesions
Response of the Periosteum (Fig. 35.2)
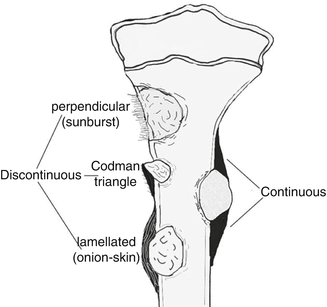
Fig. 35.2
Different types of periost reactions
May exhibit various patterns (e.g., spiculated, lamellated/onion skin, Codman triangle) [2].
The GTM staging system is commonly used in staging of skeletal tumors in which the capital “G” indicates histological grade, “T” stands for local tumor extension, and “M” represents distant metastasis. The GTM staging system is used for both benign and malignant lesions (Tables 35.4A and 35.4B).
Table 35.4A
Staging of benign bone tumors
Stage 1 | Latent | G0 | T0 | M0 |
Stage 2 | Active | G0 | T0 | M0 |
Stage 3 | Aggressive | G0 | T1–T2 | M0–M1 |
Table 35.4B
Staging of malignant bone tumors
Stage | Grade | Tumor | Metastases | Definition |
|---|---|---|---|---|
Low–grade | ||||
IA | G1 | T1 | M0 | A intracompartmental |
IB | G1 | T2 | M0 | B extracompartmental |
High–grade | ||||
IIA | G2 | T1 | M0 | A intracompartmental |
IIB | G2 | T2 | M0 | B extracompartmental |
Metastasis | ||||
IIIA–B | G1–2 | T1–2 | M1 | Any grade (A/B) distant metastasis |
The local extension and metastatic spread of suspected malignant lesions should be evaluated before biopsy; radiological staging includes a chest CT and a whole body bone scan, in addition to MRI of the primary lesion. The entire length of the involved bone should be viewed by MRI to detect satellite lesions. Biopsy is the final diagnostic step in the evaluation of skeletal malignancies, and should be performed following radiological staging and multidisciplinary assessment (orthopedic oncology, radiology, pathology, medical oncology, radiation oncology). Biopsy material can be obtained by open or closed methods (e.g., fine needle aspiration, tru-cut, trochar). It is mandatory to ascertain the exact histopathological diagnosis of any suspected malignant lesion before deciding its definitive treatment. As a general rule, it is recommended that the biopsy should be performed by the surgeon who will perform the definitive surgery.
Definition and classification of surgical margins is beneficial in planning the treatment. Most skeletal tumors are surrounded by a reactive sclerotic rim. This reactive sclerotic rim is also surrounded by a reactive zone, which includes microscopic tumor invasion. Beyond the reactive zone lies the normal tissue. High-grade sarcomas may not include a reactive sclerotic rim, a feature that indicates the aggressiveness of the lesion. Based on surgical margins, four types of surgical resections are defined: intralesional, marginal, wide, and radical (Fig. 35.3). Intralesional (the tumor is removed by disrupting its capsule or pseudocapsule) and marginal resections (the tumor is removed by a margin that may include a contaminated reactive zone) may remain macroscopic and microscopic tumor residues, respectively. Wide resection, which includes reactive zone and non-contaminated surrounding tissues, is the gold standard at local control of malignant bone lesions. Functional loss is a common complication of radical resections, in which the whole compartment including the tumor is resected [4].
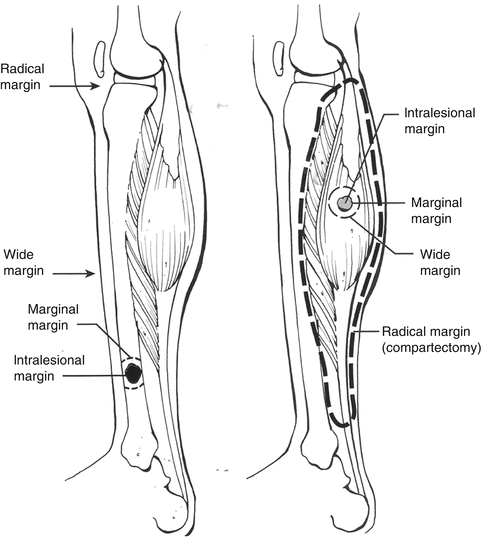

Fig. 35.3
Surgical margins. Intralesional margin, passes within the lesion; marginal, lies within the reactive zone; wide margin, passes intracompartmentally within the normal tissue; radical margin, runs extracompartmentally
Bone-Forming (Osteogenic) Lesions
Osteoid Osteoma
Osteoid osteoma constitutes 10–12 % of all benign skeletal tumors. Osteoid osteoma can be detected in any bone; however, it is most commonly observed in the femur and tibia. The most common presenting symptom is pain. Pain in osteoid osteoma is characteristic for not being relevant with motion, increasing especially during night-time and mostly relieved by aspirin and non-steroid anti-inflammatory drugs (NSAID). Lower extremity lesions may manifest with limping. Vertebral lesions may exhibit a nonstructural scoliosis.
Radiologic Features
The majority of osteoid osteomas are located intracortically and plain radiographs may indicate a dense cortical sclerosis around the lesion. A nidus, which is smaller than 1 cm in diameter, can only be noticed as a hypodense area within the dense cortical thickening (Fig. 35.4a). The best imaging modality to detect a nidus is thin-section CT (1–1.5 mm slice) (Fig. 35.4b). MRI may exhibit an exaggerated image due to the intensive edema around the lesion, resembling a malignant process. Bone scan is active at all three phases.
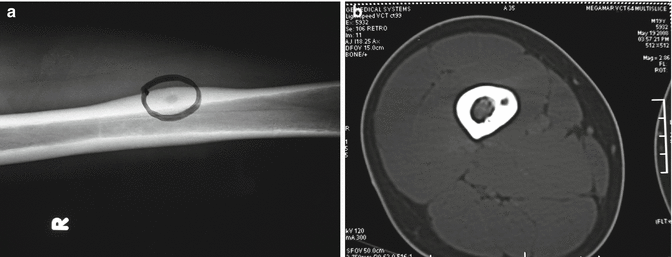

Fig. 35.4
Osteoid osteoma. (a) A hypodense nidus surrounded by a dense cortical sclerosis is seen. (b) Thin-section (1–1.5 mm) CT scan is the best imaging modality in demonstrating the nidus
Histopathological Features
Macroscopically, nidus is a red colored, hyperemic tissue that is softer than its surrounding sclerotic bone. Microscopic view shows numerous dilated capillaries and irregular woven bone seated within a fibrovascular stroma. The surrounding bone is sclerotic. Soft tissues around the lesion are mostly inflammatory.
Treatment
Surgical treatment is commonly the preferred method in the treatment of osteoid osteomas, whereas only selected cases with mild pain or with risk of complicated surgery can be managed by long-term use of NSAID. Complete removal of the nidus is mandatory in the surgical treatment of osteoid osteoma. This can be achieved by surgical resection or thermal radiofrequency ablation (RFA) [5].
Burr down is the recommended surgical method. The sclerotic bone overlying the lesion is removed level by level with a high-speed burr, until a pinhead reddish spot is seen. The nidus is curetted and underlying sclerotic bed is burred for 2–5 mm.
In CT guided thermal RFA method, the heat probe is advanced into the nidus core and heated to burn down the lesion.
Open surgical approaches warrant the removal of nidus; however, they remain disadvantageous in challenging and dangerous localizations (e.g., spinal, intraarticular, intrapelvic). CT-guided RFA, apart from being a minimal invasive procedure, is accepted as a safe method for locating the nidus precisely. Since sampling cannot be performed by CT-guided RFA, the diagnosis and treatment can only be done on the basis of clinical and radiological findings.
Osteoblastoma
Osteoblastoma is a rare bone-forming tumor, whose radiologic and histological properties resemble osteoid osteoma. This lesion is also known as “Giant Osteoid Osteoma.” The posterior vertebral arch is the predilection site for osteoblastomas, where more than 40 % of the cases arise. A progressively increasing pain not related with motion, is the most common finding. Limping may be the initial symptom in lower extremity involvement. Similarly vertebral involvement may primarily manifest as scoliosis. In plain radiographic views, lesions may present as lytic areas 2–10 cm in diameter. CT views may show intralesional calcifications (Fig. 35.5). Extended curettage and grafting is recommended for the treatment of this locally aggressive lesion. Larger lesions, vertebral and pelvic involvements, which are prone to intensive bleeding due to their rich vascularization may require selective arterial embolization prior to surgery. Risk of recurrence even after complete resection varies between 10 and 20 %.
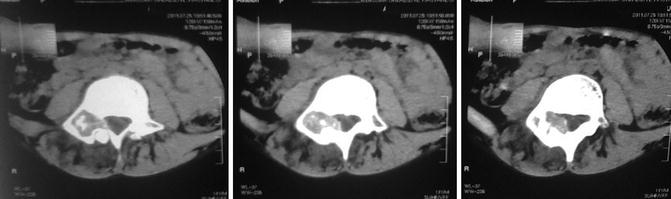

Fig. 35.5
Osteoblastoma. CT scans show a lytic lesion localized in posterior vertebral column, with intralesional punctate calcifications
Osteosarcoma
Osteosarcoma is characterized by osteoid producing malignant cells. They constitute 20 % of primary malignant skeletal tumors, being the second most common following multiple myeloma. Ninety-five percent of the cases are at the second decade of life. Ninety-five percent of osteosarcomas diagnosed in children and young adults arise as primary tumors. Secondary osteosarcomas that arise over an existing bone lesion (e.g., fibrous dysplasia), an underlying bone disease (e.g., Paget) or previous radiotherapy, are rare malignancies that mostly occur during the seventh and eighth decades of life.
High-Grade Intramedullary Osteosarcoma (Conventional Osteosarcoma)
High-grade intramedullary osteosarcomas constitute 85 % of all osteosarcoma causes. They commonly involve metaphyses of long bones where the vascularization and growth potential is exceptional. Distal femoral and proximal tibial lesions, where most of the skeletal growth is processed, constitute 50–60 % of all conventional osteosarcoma cases. Proximal humerus, proximal femur, and pelvis are the other common locations, respectively. The main complaints in admission are progressive pain and localized swelling, which are ignored or remain misdiagnosed for several months. Approximately 15 % of newly diagnosed conventional osteosarcoma patients have accompanying pulmonary metastatic disease. The presence of clinically detectable metastatic disease is a strong predictor of poor prognosis [6].
Radiologic Features
Plain radiographic views of conventional osteosarcomas are characterized by mixed lytic (radiolucent) and blastic (radiodense) activity, and mostly a metaphyseal ill-defined, destructive pattern is visible. Characteristic periosteal reaction patterns like sunburst appearance and Codman triangle and soft tissue extension, which include calcified foci, are commonly seen. Whole body Technetium Tc-99 bone scintigraphy reveals increased uptake at tumor localization. MRI is the best imaging modality that demonstrates intraosseous and extraosseous extension of the tumor and its relation with neighboring neurovascular structures (Fig. 35.6). At least one plane of the whole bone MRI is mandatory for the detection of bone marrow extension and skip lesions.
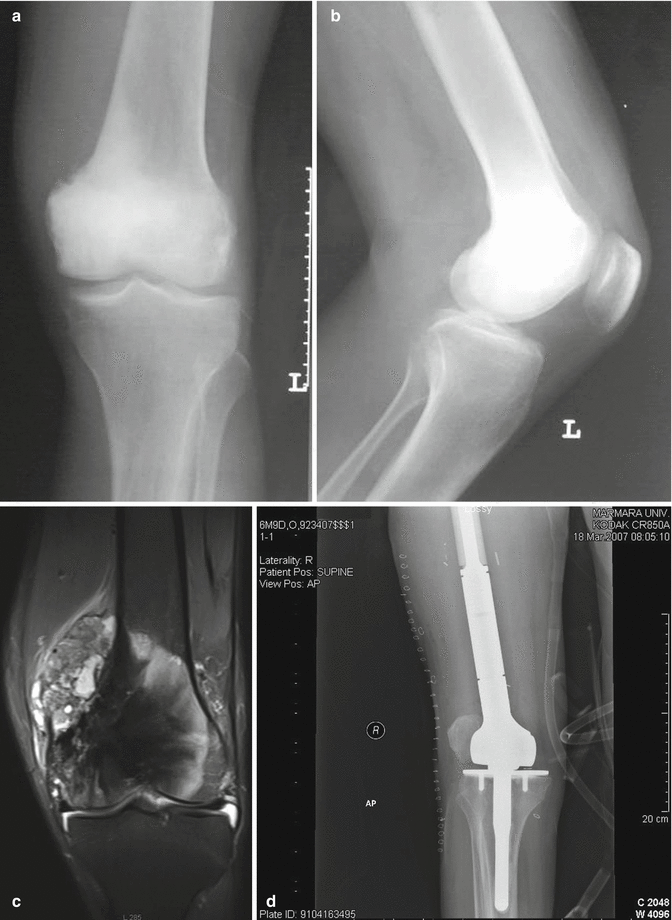

Fig. 35.6
Conventional osteosarcoma. (a, b) Plain radiographs show permeative destruction and periosteal reaction formed by tumor in distal femur. (c) Coronal MRI demonstrates intra- and extraosseous extension of the lesion. (d) Wide resection
Histopathologic Features
The osteosarcoma family, which is basically characterized by production of osteoid or new bone by neoplastic cells of mesenchymal origin, has various subtypes of similar prognostic properties. Mesenchymal neoplastic cells may differentiate to osteoblastic, chondroblastic, and fibroblastic cells. The most common subtype is characterized by pleomorphic spindle cells of osteoblastic type.
Treatment
A combination of multiagent chemotherapy and surgery is the optimal treatment of osteosarcoma. The standard approach includes neoadjuvant (induction) multiagent chemotherapy, followed by resection and adjuvant chemotherapy. Since osteosarcomas mostly are radioresistant, radiotherapy should be reserved for selected cases. A ratio of 60–75 % 5-year disease-free survival is achieved with multiagent chemotherapy and relevant surgical resection. Neoadjuvant chemotherapy aims the treatment of micrometastatic disease and reduction of tumor size by necrosis, thus enables any limb-salvage procedure. Adjuvant chemotherapy is used for the elimination of micrometastatic disease that remains after surgical resection. Response to induction chemotherapy is determined by the percentage of tumor necrosis in resected specimen. The percentage of tumor necrosis in resected specimen is evaluated histologically; a ratio over 90 % tumor necrosis indicates a good response and is correlated with higher survival rates when compared to lower tumor necrosis ratios [7].
Local control is mostly managed by surgical resection in osteosarcoma (Fig. 35.6d). Achieving tumor-free margins is the aim of wide surgical resection. Tumor-free margins can be achieved by limb-salvage surgery in most patients. Several reconstruction alternatives including endoprosthetic reconstruction, biological reconstruction, structural allografts, or allograft and prosthesis composites are available for bone defects following resections. Clavicular and fibular involvement mostly does not require reconstruction.
Approximately 50 % of osteosarcoma patients may develop local recurrence and/or late onset lung metastases [6]. The standard approach for local recurrences is mostly the radical amputation and chemotherapy. Late onset lung metastases are managed by chemotherapy with or without metastasectomy.
Surface or Juxtacortical Osteosarcoma
The term “juxtacortical” refers to a group of osteosarcomas, which are originated from the surface of the bone, and composed of parosteal, periosteal, and high-grade surface osteosarcoma variants. They are commonly encountered in the third and fourth decades of life. Parosteal osteosarcomas are low-grade osteosarcomas, which commonly arise from posterior surface of distal femur. Periosteal osteosarcomas are mostly an intermediate-grade chondroblastic type osteosarcoma and, as their name implies, commonly involve more diaphyseal localizations than parosteal osteosarcomas. Parosteal and periosteal osteosarcoma subtypes are managed by wide resection, and since both lesions are low- and intermediate-grade, their metastatic potentials are lower when compared to conventional osteosarcomas. Inadequate surgical resections are related with local recurrence. High-grade surface osteosarcomas are the least common variant of this group, which resemble conventional osteosarcomas histopathologically. Local recurrences may progress to more aggressive and high-grade lesions. High-grade osteosarcomas, like conventional osteosarcomas, require chemotherapy and surgery.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








