CHAPTER 68 Bone Substitutes
Basic Science and Clinical Applications
Spinal fusion is a common treatment for various conditions including deformity, trauma, and degenerative disc disease with instability. Over the past century, autogenous bone grafting has improved our ability to direct bone formation required for spinal arthrodesis. Nonetheless, significant rates of pseudoarthrosis up to 26% have been reported in the literature.1 Even with modern bone graft techniques and advances in internal fixation, symptomatic pseudoarthrosis still occurs in 10% to 15% of cases.2–4 Nonunion may result in poor clinical outcomes and result in extensive medical expenditure. Bone formation is essential to arthrodesis of the spine, and numerous techniques over the past decades have evolved to achieve bone formation for spinal fusion. In addition, autograft bone has certain limitations including local morbidity and limited availability. These problems have led surgeons to devise new biologic strategies, search for substitutes for autogenous bone grafting, and apply these new approaches to stimulate bone fusion.
Bone formation requires three essential components: an osteogenic potential capable of directly providing cells to the newly forming bone, osteoinductive factors that are able to signal the osteoblastic differentiation of osteoprogenitor stem cells, and an osteoconductive scaffold that assists neovascularization and supports the ingrowth of bone. The ideal bone substitute possesses all of these three properties along with an optimal biologic reaction and without risk of disease transmission. Autogenous bone grafts share these properties and are therefore considered the standard against which bone substitutes may be measured. There are, however, disadvantages with autogenous bone grafting. Autogenous iliac crest bone harvesting is associated with considerable donor site morbidity, increased operative time, and increased blood loss. Up to 30% of all patients undergoing the harvesting of iliac crest bone graft will experience significant postoperative pain or complications including infection, hematoma, nerve or vascular injury, fracture, persistent pain, abdominal herniation, or pelvic instability.3–17 Prior studies report autologous bone graft harvest to be associated with major complications in 8.6% of patients and minor complications in 20.6%.14 In addition, the amount of autograft available may be insufficient for long fusions. Similarly, revision patients may have undergone prior bone grafting procedures with little or no additional useful iliac crest bone available. For these reasons, bone substitutes may be required to augment, expand, or substitute for autogenous bone graft.
In order to avoid morbidity associated with harvesting autogenous graft and to optimize bone formation for fusion, several classes of bone substitutes have been developed. These include allografts, ceramics, demineralized bone matrices (DBMs), osteoinductive factors, autogenous platelet concentrate, mesenchymal stem cells, and gene therapy (Table 68–1). Although bone substitutes currently in clinical practice do not provide the same osteogenic, osteoinductive, and osteoconductive properties of autograft, various bone substitutes have demonstrated efficiency for bone formation in basic science and clinical studies. Advances in regional gene therapy, development of osteoinductive proteins, and production of new osteoconductive matrices herald a new era of bone biology for spine fusion.
Allografts
Allograft bone may be applied to the graft bed in a crushed particulate form or can be machined to create structural spacers. Cortical allografts offer substantial structural stability and are best suited for interbody arthrodesis. Corticocancellous allograft initially imparts little mechanical support to the fusion site but, because of its relatively large surface area, is integrated more rapidly than cortical bone.18,19 Genetic incompatibility between donor and recipient has been found to be associated with increased resorption of the allograft and histologic evidence of rejection.20
Allografts are prepared either by freezing or lyophilization (i.e., freeze-drying) in order to decrease their antigenicity and permit storage for extended periods of time.21 Frozen allografts may be stored for up to 1 year. Lyophilized allografts are dehydrated and vacuum packed, which allows for storage at room temperature. Freeze-drying reduces immunogenicity even more than freezing, but on rehydration these grafts may lose up to 50% of their mechanical strength.22 Other sterilization techniques such as ethylene oxide or radiation may compromise the material properties and osteoinductive capacity.
Despite aseptic techniques, allografts pose potential risks for bacterial contamination. In addition, there is concern for possible transmission of viral diseases such as those caused by the human immunodeficiency virus (HIV) and hepatitis virus. Nevertheless, there have been only two documented cases of human immunodeficiency virus transmission from allograft bone, both of which involved unprocessed grafts.23 The combination of rigorous donor screening and tissue processing has lowered the risk of infection to less than one per million transplants.24 Other complications observed after the implantation of structural allografts include nonunion and fracture of the graft.25 Compared with autograft, allograft incorporates slower and less completely with decreased vascularization and osteoconduction.26
Structural allografts have been used extensively for anterior interbody fusions in both the cervical and lumbar spine. Previous studies evaluating patients undergoing single-level fusions of the anterior cervical spine, with either allograft or autogenous bone, have demonstrated similar fusion rates.27,28 Structural allograft is less efficacious than autograft for promoting multilevel cervical fusions.29–31
In the posterior lumbar spine, autogenous bone graft appears to be superior to allograft for promoting posterolateral arthrodesis. In two prospective clinical trials comparing autograft with various allograft preparations, patients treated with autograft achieved solid posterolateral fusion more frequently than those receiving allograft.32,33
Jorgenson and colleagues32 conducted a prospective analysis of autografts versus allografts in posterolateral lumbar fusion in the same patient and concluded that an ethylene oxide–treated allograft is inferior to an autograft and should not be used for posterior lumbar fusions. Another prospective comparison of autografts and allografts for adult posterolateral lumbar spinal fusion reported that autografts resulted in significantly greater bone density, followed by a mixture of autografts and allografts, frozen allografts, and freeze-dried allografts.33 These reports indicate that allografts alone were not able to achieve a sufficient fusion rate for posterior spinal fusion in the adult patients. However, several studies have recommended the use of allografts as bone extenders in adolescent idiopathic scoliosis. Aurori and colleagues34 retrospectively compared the incidence of pseudoarthrosis in fusions for scoliosis supplemented with autografts and frozen allografts that were obtained from femoral heads and reported that the incidence of pseudoarthrosis was not significantly different. Dodd and colleagues35 conducted a case-control study on the use of autografts versus allografts that were from femoral heads in the surgery of idiopathic adolescent scoliosis. They reported that there was no difference, either in a radiographic assessment of bone graft mass or in the maintenance of the curve correction.
Additional studies have reported excellent outcomes with the use of femoral ring allografts in the anterior lumbar spine.36,37 In cases involving revision anterior lumbar fusions, one study found that the results obtained with tricortical allograft may be comparable with those obtained with autogenous bone graft taken from the iliac crest.38 Overall, these studies suggest that cortical allografts may be regarded as acceptable alternatives to autogenous bone graft in certain clinical situations requiring structural support and graft material such as anterior lumbar and single-level cervical fusions. Cancellous allograft may be efficacious in the adolescent patient with scoliosis undergoing fusion and may also be used to supplement a limited quantity of autograft for posterolateral arthrodesis.
Ceramics
Ceramics are bone substitutes designed to be osteoconductive to ingrowth of new bone.39 The most commonly used ceramic scaffolds for spinal fusion are calcium phosphates such as hydroxyapatite, tricalcium phosphate (TCP), and a combination of these materials. Ceramics are favorable bone substitutes because they are biodegradable, nontoxic, nonimmunogenic, easy to sterilize, and available in virtually unlimited supply without donor site morbidity or infection risk. Disadvantages of ceramic structures are their brittle structure and reduced shear strength and resistance to fracture. Because they offer minimal mechanical stability in the immediate postoperative period, ceramics are commonly used in conjunction with rigid internal fixation and must be protected from loading forces until they are incorporated into the surrounding bone.
In general, ceramic scaffolds can be used as bone graft extenders to expand an existing quantity of available local autograft bone chips for posterolateral spinal fusion. With rigid instrumentation, several studies have reported that ceramic scaffolds are efficient bone graft extenders in posterolateral spinal fusion.40–42 Although ceramic scaffolds appear to be suitable bone graft extenders, hydroxyapatite alone may be insufficient for intertransverse posterolateral fusion. An adequate vascularized bone surface such as decorticated lamina may be required for incorporation of coralline hydroxyapatite mixed with local bone and bone marrow. As such, iliac bone autografts remained the gold standard for achieving solid posterolateral fusion.43
On the other hand, successful results have been reported for the implantation of ceramic scaffolds for posterior spinal fusion in scoliosis cases, which require extensive bone graft.44,45 Ransford and colleagues46 conducted a prospective randomized study to evaluate the use of a synthetic porous ceramic as a bone graft substitute in posterior spinal fusion for idiopathic scoliosis; they concluded that porous ceramic is a safe and effective bone substitute. Tricalcium phosphate may be an alternative to allograft as an extender when large volumes of graft are necessary in posterior spinal fusion for scoliosis.47 For anterior spinal fusion, ceramic scaffolds need to be used with rigid internal fixation. Thalgott and colleagues48–49 reported a retrospective study to evaluate the efficacy of coralline hydroxyapatite as a bone replacement in anterior interbody fusion in both the cervical and lumbar spine. They concluded that the use of coralline hydroxyapatite with rigid anterior plating appeared to be a promising bone replacement in anterior fusion, but it was not recommended for stand-alone anterior interbody fusion.48–49
The bioresorbability of a ceramic is influenced by the shape, density, and chemical composition of the material. Hydroxyapatite is a relatively inert substance that is retained in vivo for prolonged periods of time, whereas the more porous tricalcium phosphate typically biodegrades in about 6 weeks.50 There is some concern that ceramic particles may provoke an inflammatory response that could eventually bring about the significant resorption of bone, similar to the osteolysis triggered by debris from total joint prostheses, but at this time there is little evidence substantiating this risk. Although the implantation of ceramics alone has been associated with successful outcomes after anterior cervical intervertebral fusion51 and posterior spinal fusion for adolescent idiopathic scoliosis,52,53 these osteoconductive scaffolds are usually coupled with other osteogenic or osteoinductive materials. When loaded with a source of osteogenic cells such as autogenous bone or bone marrow, ceramic scaffolds assist cellular adhesion, support vascular ingrowth, and promote new bone formation.54 Ceramic carriers may also function as effective vehicles for the delivery of osteoinductive growth factors.
Demineralized Bone Matrices
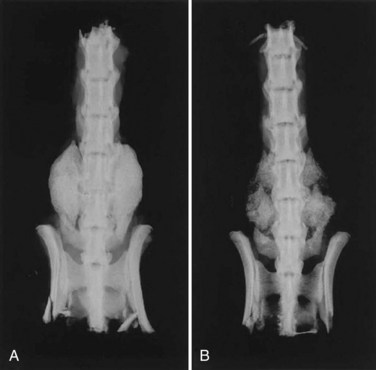
DBMs are derived from allograft bone by removing the mineralized component but preserving type I collagen and noncollagenous proteins including numerous growth factors. The osteoinductive properties of DBMs were first recognized by Urist55 in 1965, when he reported that the introduction of decalcified bone brought about the formation of heterotopic bone in rodents. The bone morphogenetic proteins (BMPs) represent less than 0.1% of all bone proteins by weight,56 but these growth factors are essential to the process of osteoinduction, initiating a cascade of cellular events leading to bone formation. Commercially available DBMs have demonstrated marked variability in osteoinductive potential that may reflect differences in their BMP content in rat spinal fusion models (Fig. 68–1).57–59
Posterolateral spinal fusion may be successful using DBMs alone or in conjunction with autograft in a rabbit and nonhuman primate model.60–63 Clinical studies also support the efficacy of DBMs as bone graft extenders for posterolateral spinal fusion.64,65 A composite consisting of DBM putty and aspirated bone marrow offers a similar performance as autograft in posterolateral spinal fusion. A multicenter prospective study compared the effectiveness of a Grafton (Osteotech; Eatontown, N.J.) DBM gel composite with iliac crest autograft in posterolateral spinal fusion; this demonstrated that Grafton DBM could extend a volume of autograft that was less than normally required to achieve a solid spinal fusion.66 DBM and bone marrow composite has been successful for posterior spinal fusion in scoliosis cases, and the fusion rates have been reported to be comparable with those of autograft.67 In a case series of anterior lumbar interbody fusions with DBM composites consisting of titanium mesh cages, coralline hydroxyapatite, and DBM, one study concluded that the DBM composite was effective for anterior interbody fusion of the lumbar spine when used as part of a rigidly instrumented circumferential fusion.68 On the other hand, An and colleagues69 prospectively analyzed the fusion rates of an allograft-DBM composite, as compared with autograft, in anterior cervical fusion and concluded that the allograft-DBM construct resulted in a higher rate of graft collapse and pseudarthrosis than autograft alone.
In a direct comparison of multiple formulations of the same DBM, the putty and flexible sheet forms enhanced spinal fusion to a greater extent than a gel, most likely because the former are fiber-based preparations and exhibit improved handling characteristics over the putty form.70 Because they do not contain viable cells, DBMs are most effective when implanted in environments that offer suitable vascularity and an adequate supply of osteoprogenitor cells. In an attempt to further augment bone formation, autogenous bone graft or bone marrow aspirates are often added to DBMs in order to increase the number of osteogenic cells able to respond to the osteoinductive growth factors. DBMs provide minimal structural support and are generally implanted into locations where they are not subjected to excessive biomechanical forces. The osteoinductive potential of DBMs has been tested in both intervertebral (interbody) and posterolateral spinal fusion models in a wide range of animals. DBMs have been found to promote successful arthrodesis of the spine when used alone or in conjunction with autograft, bone marrow, or ceramics.71–73
Osteoinductive Growth Factors
Numerous growth factors capable of signaling cell-surface receptors and directing cellular activities are involved in the regeneration of new bone tissue. In spinal surgery, growth factors critical to embryonic bone formation and fracture healing are powerful adjuvants to obtain fusion. In 1965 Marshal Urist55 first observed that DBM possessed osteoinductive ability; subsequently, the osteoinductive BMPs were isolated and characterized. BMPs are members of the transforming growth factor-beta (TGF-β) superfamily. By binding to specific receptors present on the surface of the osteogenic progenitor, intracellular cascades that recapitulate endochondral ossification are activated. BMPs stimulate mesenchymal stem cells to differentiate. Early BMP extracts were acquired in a partially purified form using techniques that called for large amounts of bone; with these inefficient methods, 10 kg of cortical bone yielded less than 20 g of osteoinductive protein.74 In addition, these crude preparations contained a heterogeneous collection of growth factors including several different types of BMPs, as well as other biologically inactive proteins.
Taking advantage of advances in molecular and cellular sciences, the genes encoding the BMP proteins were sequenced and subsequently cloned, allowing for the mass production of a single BMP including BMP-2 and BMP-7 (also known as osteogenic protein-1 [OP-1]).75 Because they are available in almost unlimited quantities, BMP-2 and BMP-7 have become the most widely used recombinant BMPs for animal studies and are the only BMPs currently being evaluated in human clinical trials. In contrast to purified extracts, recombinant growth factors are free of impurities and do not elicit a host immune response. Recombinant human BMPs (rhBMPs) are soluble factors that tend to diffuse away from the fusion site when used alone, resulting in attenuation of their osteoinductive capacity. For this reason, before implantation, these factors are combined with a carrier matrix that serves to restrict their movement, confine them to the location where they are needed, and allow them to release consistently over time. These substrates may also act as osteoconductive scaffolds that support new bone formation by promoting cellular adhesion and angiogenesis. Autogenous bone graft, DBMs, collagen, ceramics, and polylactic acid (PLA) have all been used to deliver rhBMPs, but at this time the ideal carrier has not been identified.75–87 It is likely that the most suitable method of delivery may be dependent on the specific clinical application being treated and the location into which the growth factors will be introduced. Once these growth factors have been distributed to the area of interest, osteogenic cells that are able to respond appropriately to these osteoinductive proteins must also be present for any significant bone production to occur. Multiple animal studies, many of which were performed in nonhuman primates, have established that the implantation of rhBMPs such as BMP-2 and BMP-7 in the posterior spine results in fusion rates equivalent or superior to those obtained with autogenous bone graft. Furthermore, they may generate fusion masses with improved biomechanical properties that may obviate the need for decortication of the posterior elements, a procedure that is normally required to provide endogenous growth factors that are essential for the successful arthrodesis of the posterolateral spine.88 Boden and colleagues89 conducted a prospective randomized clinical pilot study on the use of rhBMP-2 for posterolateral fusion in humans. In that study, the authors randomly divided the enrolled patients into three treatment groups as follows: autograft with instrumentation, rhBMP-2/ ceramic granules with instrumentation, and rhBMP-2/ceramic granules only without instrumentation. They reported that the fusion rate of the rhBMP-2/ceramic granules without instrumentation group was 100%, which was superior to the autograft with instrumentation group (40%).89 Following this pilot study, Dimar and colleagues90 conducted a prospective randomized study comparing the use of iliac crest bone graft to rhBMP-2 combined with a carrier consisting of bovine collagen and tricalcium/hydroxyapatite for single-level posterolateral fusions. Those authors also reported that the rhBMP-2 group demonstrated increased fusion rates as compared with the autograft group.90 In a prior study Boden and colleagues91 described the human pilot trial of the use of rhBMP-2/collagen inside lumbar interbody spinal fusion cages. Although the number of patients enrolled in that study was small, they reported at the 2-year follow-up that fusion occurred more reliably in patients treated with rhBMP-2-filled cages than in controls treated with autogenous bone graft.91 Burkus and colleagues also conducted a prospective study on the use of rhBMP-2/collagen sponge with allograft dowels or tapered cylindrical fusion devices in anterior lumbar interbody fusion and concluded that the use of those rhBMP-2 composites showed promise in assisting anterior intervertebral spinal fusion.92–94 Slosar and colleagues,95 in a prospective study on anterior lumbar interbody fusions, compared patients treated with allografts, either with or without the addition of rhBMP-2, with posterior instrumentation and demonstrated excellent results with the use of rhBMP-2. These reports supported the use of rhBMP-2 for anterior lumbar interbody fusion. On the other hand, there are reports that rhBMP-2 can cause aggressive resorption of an implanted graft before osteoinduction and interbody fusion occurs. McClellan and colleagues96 retrospectively investigated cases with a transforaminal lumbar interbody fusion with BMP; they reported a high rate of bone resorption defects and assumed that the osseous remodeling potential of rhBMP-2 may lead to bone resorption within the vertebral body. Pradhan and colleagues97 reported that the pseudarthrosis rate among patients who received femoral ring allografts with rhBMP-2 was higher than that in patients who received femoral ring allografts with autogenous iliac bone. They concluded that this appeared to be caused by the aggressive resorptive phase of allograft incorporation, which occurs before the osteoinduction phase.97 These results suggest that caution must be exercised in deciding between autograft and rhBMP-2 for anterior lumbar interbody fusion and that further clinical studies are warranted.
It appears that the osteoinductive activity of the BMPs may compensate for the inhibitory effects of nicotine and nonsteroidal anti-inflammatory drugs (NSAIDs), two agents implicated in hindering spinal fusion in humans.98–102 As noted previously, recombinant growth factors have been used in conjunction with intervertebral fusion devices in an attempt to achieve arthrodesis of the anterior spinal column. Titanium cages or cortical allograft dowels loaded with rhBMPs proved to be more efficacious than similar devices carrying autogenous bone for stimulating interbody fusion in a number of different animal models, a finding that was consistent whether these composite grafts were implanted in the cervical, thoracic, or lumbar spine.101–103 These encouraging results were corroborated by a prospective, randomized clinical trial in which patients with degenerative disc disease limited to a single level of the lumbar spine were treated with a threaded cylindric cage filled with either rhBMP-2 protein or autograft.104 After 24 months, all 11 patients who had received rhBMP-2 exhibited radiographic evidence of solid fusion, compared with only two of the three control patients who were implanted with interbody devices containing their own iliac crest bone. The rhBMP-2 group initially experienced a more rapid resolution of their original symptoms, although at 6 months both groups demonstrated similar levels of clinical improvement. In addition, no complications were reported with the use of rhBMP-2. These studies have confirmed that partially purified BMP extracts and recombinant growth factors are able to induce spinal fusion in animals and humans. In all of these studies, however, the concentration of BMP necessary to bring about adequate bone formation in this environment was several magnitudes of order greater than normal physiologic levels, an observation that raises potential safety concerns. In a canine lumbar spine fusion model, the placement of osteogenic protein-1 (OP-1) over a dural tear stimulated new bone formation in the subarachnoid space, resulting in mild spinal stenosis at the site of dural decompression.105 Before osteoinductive growth factors may be used clinically as a bone graft substitute, they must be subjected to further testing to ensure that the introduction of milligram doses of these proteins into patients is not associated with any significant immunogenicity, toxicity, or other adverse effects.
In contrast to anterior lumbar fusion, there are studies that caution against the use of high-dose rhBMP-2 for cervical anterior spinal fusion. Shields and colleagues107 reported a retrospective review of patients who underwent anterior cervical fusion using high-dose rhBMP-2/collagen sponge. The authors reported that 23.2% of patients suffered complications such as hematomas, dysphagia, and excessive edema. Vaidya and colleagues108 also reported that complications were associated with anterior cervical spinal fusion using rhBMP-2 including dysphagia that was shown to be significantly more frequent and more severe in patients in whom rhBMP-2 was used. As a consequence, in July 2009 the U.S. Food and Drug Administration issued a Public Health Notification citing serious adverse effects with use of BMP in the cervical spine. Therefore rhBMP-2 must be used cautiously for anterior cervical spinal fusions until more research is undertaken and these clinical issues are resolved. Because the administration of large amounts of BMP is expensive, economic analyses should be completed to determine the cost-effectiveness of using growth factor therapy as a substitute for autogenous bone graft in the spine. In order to minimize the quantities of BMP required for a successful fusion and outcome, it will be important to establish the appropriate dose for each spinal application and develop efficient carrier systems to deliver these osteoinductive factors. Finally, it is important to note that, in the spine, BMP is FDA approved only as an adjunct with a threaded lumbar interbody cage. Other uses are considered an off-label use.
New adjuvant agents such as bone morphogenetic binding peptide (BBP), which may enhance the efficiency of BMP, are under investigation in animal and in vitro studies. BBP is a BMP-specific binding protein that was isolated on the basis of the early work of Urist. BBP binds rhBMP-2 with an intermediate affinity, which makes it an ideal “slow release” agent.108 As such, BBP may reduce the time to fusion and more thoroughly control the distribution of bone healing in spinal fusion.