Fig. 10.1
(a) A pictorial review of the Genant semiquantitative method for vertebral fracture assessment: (a) Anterior wedge fracture: measure the anterior height loss relative to posterior height (i.e. ratio = (P−A)/P × 100 %). (b) Biconcave fracture: measure the minimum central vertebral body height relative to posterior height (i.e. ratio = (P-M)/P × 100 %). Note that this fracture description is a misnomer because in many cases only one endplate is involved; the term is retained for fidelity with the original Genant grading system. (c) Posterior crush fracture: this height should be measured relative to the posterior height of the adjacent vertebrae because the posterior body wall is altered in this type of fracture and cannot serve as its own reference. The lower of the two ratios (Phi–P)/Phi and (Plo–P)/Plo is recorded as a percentage. Because L4 is the lowest level scored in Genant, at L4 only the L3 posterior height is used for comparison. Genant grades are: 0, height loss 20 % or less; 1 (mild), >20–25 %; 2 (moderate), >25–40 %; 3 (severe), >40 %. (b) Examples of actual fractures of each subtype in the Genant semiquantitative grading system. All images are sagittal radiographs of thoracolumbar vertebrae oriented with anterior to the right. Top row: 5-year-old girl with acute lymphoblastic leukemia; 9-year-old girl with acute lymphoblastic leukemia; 6-year-old boy with acute lymphoblastic leukemia. Middle row: 10-year-old girl with acute lymphoblastic leukemia; 10-year-old girl with osteogenesis imperfecta; 5-year-old girl with acute lymphoblastic leukemia. Bottom row: 9-year-old girl with acute lymphoblastic leukemia; 8-year-old girl with acute lymphoblastic leukemia; 10-year-old boy with histiocytosis. Arrows indicate the fracture in each image. In the image of the grade 2 crush fracture, note also the grade 1 wedge fracture at the vertebra below (with permission from Jaremko et al. and the Canadian STOPP Consortium, Pediatr Radiol, 2015)
A number of recent studies have provided validity for the Genant approach in children given the following key observations. First, Genant-defined VF in children give rise to a bimodal distribution of fractures from T4 to L4 similar to the known distribution in adults [7–10], with a predilection for the mid-thoracic region (T5 to T8, the site of the natural kyphosis) and the thoracolumbar junction (the site of the natural lordosis) [1, 10] (Fig. 10.2). Secondly, a number of biologically relevant clinical predictors of Genant-defined VF have been identified, including back pain, low lumbar spine (LS) BMD Z-scores, longitudinal decreases in LS BMD Z-scores, and GC exposure [1, 11, 12].


Fig. 10.2
(a) The frequency and location of vertebral fractures in children with acute lymphoblastic leukemia at diagnosis in relation to the number of fractures identified as mild (grade 1), moderate (grade 2), and severe (grade 3) at each vertebral level. Most vertebral fractures occur in the mid-thoracic region and at the thoraco-lumbar junction. (b) The morphology of vertebral fractures in relation to location and frequency among children with ALL at diagnosis (an anterior wedge fracture is the most common morphology in children) (with permission from Halton et al. and the Canadian STOPP Consortium, J Bone Min Res, 2009)
A key observation to assert the validity of this approach in children is that Genant-defined VF at leukemia diagnosis are a robust clinical predictor of incident (new) VF in the 3 years following chemotherapy initiation [12, 13]. Even grade I (mild) VF at GC initiation independently predict incident VF over the ensuing 3 years in children [12, 13]. This observation lends credence to the cut-off of at least 20 % loss of vertebral height ratio to define a pediatric VF by the Genant method. Gaca et al. [14] have shown that 95 % of healthy children had anterior wedging at the thoracolumbar junction that did not exceed an 11 % reduction in anterior:posterior height ratio. This observation is consistent with the traditional radiology teaching that normal physiological rounding of vertebral bodies, which is frequently observed in children prior to the appearance of the ring apophysis around the time of puberty, does not exceed about 10 % [15]. Taken together, the critical threshold of a 20 % loss in vertebral height ratio to define a VF in children appears valid, and is increasingly used in clinical practice, research studies, and clinical consensus statements [16].
A third VF assessment method has been developed, which addresses the issue that quantitative or semiquantitative morphometry does not fully capture other important signs of VF, including endplate deformity (interruption), anterior cortical buckling, and loss of endplate parallelism (with loss of vertical continuity along vertebral bodies anteriorly). The “algorithm-based qualitative (ABQ)” method developed by Jiang et al. [17] is based on the assumption that the vertebral endplate is always deformed in the presence of a VF. This method asserts that the endplate is always centrally depressed (whether concave, wedge, or crushed), and that vertebral height ratios can be diminished in non-VF states such as oblique image projections and with certain anatomical variants (such as Cupid’s bow deformity). The ABQ method uses a flowchart to systematically rule out non-VF deformities that mimic VF by examining various distinct radiological characteristics of the vertebral bodies. A radiologist skilled in the ABQ method is needed to differentiate accurately between VF and non-VF deformities or normal variants. The Canadian STeroid-induced Osteoporosis in the Pediatric Population (STOPP) Consortium, a national pediatric bone health working group that was established to understand the natural history of VF in the pediatric GC-treated setting, recently published an atlas of non-VF deformities and normal variants in children which can mimic VF. This atlas serves as a valuable resource to radiologists and clinicians who assess spine radiographs in children [18] (Fig. 10.3).
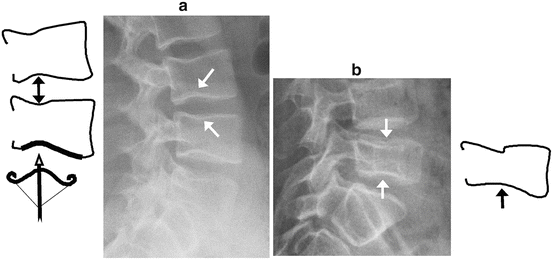

Fig. 10.3
Normal variants mimicking fractures in children with glucocorticoid-treated diseases. (a) Cupid’s bow vs. biconcave fracture: Diagram and lateral radiograph depict Cupid’s bow or balloon disk at multiple levels in a 17-year-old boy treated with glucocorticoids for vasculitis. This normal variant is a curved indentation centered at the posterior third of the endplate (arrows). The shape resembles Cupid’s bow (diagram, bottom), and when present at adjacent endplates it gives the impression of disk expansion, hence it is also known as “balloon disk.” (b) Biconcave endplate fracture at L5, in a 10-year-old boy treated with glucocorticoids for acute lymphoblastic leukemia. There is interruption of the superior endplate of L5, the endplate concavities are centered at the mid-disk rather than the posterior third, there is overall height loss, and adjacent levels are not affected (with permission from Jaremko et al. and the Canadian STOPP Consortium, Pediatr Radiol, 2015)
Overall, the Genant semiquantitative method remains in widest use, including in children. The Genant method has the advantages that it quantifies the severity of VF (an important predictor of the potential for spontaneous vertebral body reshaping following VF in children) and it permits calculation of the SDI (a measure of the child’s global spine morbidity as discussed). The kappa statistics for intra- and inter-observer agreement are similar for children compared to adults using the Genant semiquantitative method [5, 19, 20]. In general, the ABQ method shows low to moderate agreement with other methods [17].
Imaging Methods for Vertebral Fracture Detection
To date, the most common imaging tool for VF detection in childhood (also the technique upon which the Genant semiquantitative method was pioneered) has been lateral thoracolumbar spine radiographs from T4 to L4. These vertebral levels are typically targeted since T1 to T3 can be difficult to visualize due to overlying lung, and L5 has normal posterior wedging that can be confused with a fracture. However, if T1 to T3 are well-visualized and more than 20 % loss of vertebral height ratio is observed, then this finding should also be reported by the radiologist.
In view of the high radiation exposure from spine radiographs, nonradiographic imaging techniques have been developed which use the scoring methods described above. One such technique is “vertebral fracture assessment” (VFA), using images captured on a lateral spine DXA. VFA is extremely attractive as a VF assessment tool, given its low radiation and the fact that fan-beam technology facilitates capture of the entire spine on a single image without divergent beam issues due to parallax. Newer DXA machines have a rotating “c-arm” which obviates the need to re-position the patient from the supine to the lateral position. Recently, it has been shown that image quality varies significantly depending on the densitometer [21]. Using a Hologic Discovery A machine, Mayranpaa et al. [22] showed low diagnostic accuracy for VFA compared to lateral spine radiographs and suboptimal image quality for children. Specifically, only 36 % of VF identified on lateral spine radiographs were evident by VFA. Pediatric studies on newer DXA machines to determine the validity of this approach are presently underway [23]. Since the prevalence and incidence of VF in children with various osteoporotic conditions is clinically significant, validation of DXA-based VFA in the pediatric population is a meritorious endeavor.
Magnetic resonance imaging (MRI) is a three-dimensional evaluative tool without ionizing radiation that can demonstrate bone marrow edema, and is therefore useful in distinguishing acute versus old fractures. The disadvantage of MRI detection of VF is the long imaging time and high costs. Images produced by computed tomography (CT) have much higher spatial resolution than MRI and DXA; however, it has been shown that sagittal reformations are needed to demonstrate VF [24] and the dose of radiation is substantial with CT.
The Epidemiology of Vertebral Fractures in Pediatric Osteoporotic Conditions
A number of studies have underscored that VF are an important, yet frequently over-looked manifestation of osteoporosis in children. This is particularly true in children with OI and in children with GC-treated disorders given the predilection of GC therapy to adversely impact the trabecular-rich spine. At the same time, children who are GC naïve are not exempt from VF, as the VF prevalence has been shown; for example, 25 % of children with motor disabilities have detectable VF [25].
Pediatric VF frequently go undetected in this population for two main reasons. First, VF are often asymptomatic in children [1, 12, 26–29], even in the presence of moderate and severe collapse [1]. Secondly, routine surveillance in at-risk children with a spine X-ray has not, to date, been signaled an important element of osteoporosis monitoring guidelines. However, the 2014 guidelines developed by the International Society for Clinical Densitometry have stated that the diagnosis of osteoporosis can be made in children with at least one vertebral fracture without BMD criteria [16], thereby advocating that spine health monitoring beyond BMD is needed in at-risk children.
In adults, only about one third of all VF come to clinical attention and 2/3 go undetected [30]; this observation is consistent with reports in children [1, 12, 27, 29]. The importance of identifying even asymptomatic VF is evident in the adult literature since both symptomatic and nonsymptomatic VF are associated with decreased quality of life [31, 32] and increased mortality [33]. While similar studies have not been carried out in children to determine the relationship between VF and quality of life measures or mortality risk, it is conceivable that this might be true in younger patients. Large, prospective studies are needed to investigate these relationships in more detail.
Osteogenesis Imperfecta
The severity of bone fragility in OI spans a broad spectrum, ranging from asymptomatic individuals to perinatal lethality. In most cases, OI is caused by autosomal dominant mutations in either the COL1A1 or COL1A2 genes, interfering with either the synthesis or the structure (or both) of type I procollagen alpha1 or alpha2 chains [34]. Less often OI is due to recessive mutations, most of which interfere with post-translational processing and trafficking of type I procollagen [34].
VF are one of the hallmarks of OI, present in most patients with severe forms and a large proportion of those with the milder type I OI. Despite the common occurrence of VF in OI, there are few data on the prevalence of VF and none reporting the incidence of VF during the pediatric years. Ben Amor et al. [35] recently studied a large cohort of children with OI due to COL1A1 haploinsufficiency. Haploinsufficiency results in about one-half of the normal amount of collagen type I protein. Mutations giving rise to haploinsufficiency are associated with a milder form of OI characterized by normal or near-normal stature and milder degrees of long bone and craniofacial deformity. However, 41 of 58 children (71 %) had VF on lateral spine radiographs [35]. The authors noted that this proportion does not represent a true prevalence, since some patients only underwent spine radiographs in the setting of back pain. Nevertheless, these estimates suggest the true prevalence is likely to be high. Girls had a median of 4 VF (range 0–14) while boys had a median of 1 VF (range 0–8), despite the fact that LS BMD Z-scores were slightly higher in girls compared to boys. The higher BMD in girls with VF and OI may be related to the fact that, according to trans-iliac histomorphometry studies, bone turnover is lower in girls compared to boys [36].
Childhood Leukemia
Skeletal morbidity has long been associated with acute lymphoblastic leukemia (ALL ), occurring at diagnosis , during treatment, and in the years after therapy [1, 12, 37]. Since current leukemia therapy results in a cure rate that approaches 80 % [38], ALL represents a transient threat to bone health for most children and adolescents . Although many children with skeletal morbidity with ALL show evidence of recovery [39], some are left with permanent skeletal abnormalities [40]. This underscores the importance of identifying those at risk for long-term sequelae and implementing appropriate treatment and prevention strategies.
At diagnosis, skeletal abnormalities are evident on plain radiographs in up to 75 % of children [41]. They include metaphyseal lucencies from aggressive osteolysis [42–44], periosteal separation, metaphyseal growth arrest lines and pathological extremity fractures [41–44]. Based on data from retrospective [41, 45, 46] and prospective [47] studies, the prevalence of low trauma non-VF ranges from 3 to 10 %. VF can cause a limp or inability to walk as a presenting feature of the disease. Incident VF also occur frequently around the time of diagnosis in childhood ALL; a prospective VF surveillance study confirmed that the spine fracture prevalence is 16 % at this time point [1]. Bone fragility at the time of ALL diagnosis has been linked to increased bone resorption resulting from cytokines released by the leukemic cells causing osteoclast hyperactivity [48].
The incidence of VF (both asymptomatic and symptomatic) is highest in the first year of chemotherapy for ALL; 16 % of patients developed VF within the first 12 months of chemotherapy [13]. The 4-year cumulative VF incidence in ALL was 35 % in one study. Over half of the patients reported an absence of back pain in the 12 months preceding the annual spine surveillance radiographs for fracture detection [13].
Glucocorticoid-Treated Inflammatory Disorders
Children with rheumatic disorders are also fracture-prone, both at diagnosis and following GC initiation. In rheumatic disorders, the prevalence of VF at the time of GC initiation is 7 %, with a prevalence ranging from 10 to 23 % after long-term GC therapy [49–51]. The rate of incident VF ascertained by annual spine radiograph surveillance was 6 % after 12 months of GC treatment in a large cohort of children with a variety of rheumatic disorders; 40 % of the children with incident VF had juvenile dermatomyositis [29]. The 3-year cumulative VF incidence following GC initiation increases to 13 % [11], and those with juvenile idiopathic arthritis have a 50 % to threefold increased risk of a fracture (all types) during the pediatric years [52]. In a prospective study of children with a variety of GC-treated rheumatic disorders, one child with a normal lateral spine radiograph at GC initiation presented with painful, grade 1 and 2 VF after only 4 months of GC therapy, highlighting that overt bone morbidity can be evident soon after GC initiation [29] (Fig. 10.4). In keeping with other recent observations that VF are an under-recognized but nevertheless discrete manifestation of osteoporosis in children with inflammatory disorders [27, 29, 50, 53], VF occurred more frequently in an 80 patient cohort of children and adolescents with inflammatory bowel disease (11 % prevalence after an average of 3 years from diagnosis, compared to 3 % in healthy controls, p = 0.02).


Fig. 10.4
Spine radiographs from a 7-year-old girl with mixed connective tissue disease. At study entry, (a) her spine radiograph showed no signs of vertebral fractures; however, she manifested multiple painful vertebral fractures after only 4 months of glucocorticoid therapy. (b) This patient’s clinical course was distinguished by Cushingoid features and a dramatic increase in body mass index (by 3.1 SD) in the first 3 months of glucocorticoid therapy. This was despite similar glucocorticoid exposure compared to the other children with rheumatic diseases and incidence VF in the first year following glucocorticoid initiation. An increase in body mass index Z-score in the first 6 months of glucocorticoid therapy is a known predictor of incident vertebral fractures over the following 3 years (see Table 10.1) (with permission from Rodd et al. and the Canadian STOPP Consortium, Arth Care Res, 2012)
Neuromuscular Disorders
GC-treated Duchenne Muscular Dystrophy (DMD) is a “perfect storm” for severe osteoporosis with the combined threats of progressive myopathy and GC therapy. VF in pediatric DMD can be associated with severe chronic back pain; in addition, both spine and leg fractures can lead to premature, permanent loss of ambulation. While the annual incidence of fractures remains unknown, a few reports describing estimates of VF prevalence in pediatric DMD have been carried out [54–56]. These cross-sectional studies have shown that up to 30 % of boys with DMD develop symptomatic VF, and 20–60 % sustain extremity fractures. The fact that only symptomatic VF have been reported represents a knowledge gap in pediatric DMD, since we know that VF are frequently asymptomatic [12, 29]. Whether asymptomatic or not, VF are strongly associated with an increased risk of future fractures [12].
Children with cerebral palsy represent another group with an increased risk of VF. The overall prevalence of fractures (all types) in this condition is reported between 4 and 12 % [57]. A cross-sectional study of children with cerebral palsy and Gross Motor Function Classification System scores II or higher who underwent lateral spine radiographs showed that 7/37 (19 %) had VF; the back pain status in these patients was not reported. Since children with cerebral palsy are often nonverbal, even symptomatic VF may go undetected in this population.
Nephrotic Syndrome
In contrast to pediatric ALL, DMD and rheumatic disorders, GC-treated nephrotic syndrome is considered an in vivo model of the impact of GC therapy on bone, since the underlying disease itself is not known to have a deleterious effect on the developing skeleton. Two studies have evaluated the prevalence and incidence of VF in GC-treated nephrotic syndrome [26, 28]. The first prospective study found the prevalence of VF within 37 days of GC initiation was 8 %, all of which were mild and asymptomatic [26]. There was an inverse relationship between GC exposure and LS BMD Z-scores even after just 37 days of GC therapy, testament to the expected rapidity with which GC exert their effect [58]. The second prospective study reported a 6 % incidence of VF after 12 months of GC therapy; LS BMD Z-scores improved in the majority by 12 months as many children entered remission and discontinued GC therapy. A disproportionate number of children had LS BMD Z-scores ≤1.0 at 12 months; further study of these children revealed that their lower LS BMD Z-scores at 12 months were inversely associated with GC exposure in the first 3 months of therapy, despite similar GC dosing compared to the rest of the cohort. This observation suggests that some children are more sensitive to the osteotoxic effects of GC therapy compared to others, possibly reflecting that polymorphisms in the GC receptor gene are associated with GC dependence in pediatric nephrotic syndrome [59].
Organ Transplantation
In children with hepatic diseases requiring transplantation, the overall fracture prevalence in retrospective studies ranges between 10 and 40 % of children prior to transplant [60–66] and from 12 to 50 % following transplant [60, 62, 63, 67]. A recent retrospective study of 40 children and adolescents post-liver transplant assessed patients’ VF statuses through VFA on a Hologic machine (model not specified) [60]. A lateral spine radiograph was performed in those with spine BMD Z-scores ≤−2 SD, those with VFA images suggesting a VF, or when vertebral bodies were not sufficiently visible by VFA. With this approach, 73 % of patients required lateral thoracolumbar spine radiographs in addition to VFA and 7/40 patients (18 %) had prevalent VF. A study of pediatric liver, kidney, and bone marrow transplant recipients [62] reported a sixfold higher fracture rate for all fracture types and a 160-fold higher rate of VF compared to controls. Fifty percent of the pediatric patients with VF in this series were asymptomatic, consistent with the high frequency of asymptomatic VF in other pediatric chronic illnesses [1, 12, 29].
Bone Mineral Density and Other Clinical Predictors of Vertebral Fractures
As shown in Table 10.1, a number of studies have been sufficiently powered to assess clinical predictors of VF in bi- or multivariable models or through nonparametric analyses. Most of these studies have been retrospective or cross-sectional; very few studies have assessed the frequency of incident VF in relation to the evolving clinical course of the child. A detailed description of the clinical predictors in the various disease states according to the published literature is provided in Table 10.1, along with the criteria for carrying out a VF assessment in a given population of children, the method and imaging modality for VF detection, and the overall incidence and prevalence rates. Note that if specific clinical criteria triggered VF assessment (such as a low BMD or back pain), then the estimate of VF prevalence or incidence in that cohort was only relevant to children with the same clinical profile. These heterogeneous methodologies need to be taken into account when comparing prevalence and incidence rates across studies with similar disease groups.
Table 10.1
Relationship between clinical predictors and prevalent/incident vertebral fractures
Disease | Publication and study design | Inclusion criteria | Number, age (yrs), and male (%) of patients | Clinical criteria for carrying out the VF assessmenta | VF assessment method and imaging modality | VF prevalence/incidence and time point | Clinical predictors of prevalent or incident VF from univariate or multivariable models (with effect size and 95 % CI) or statistical tests for comparing children with and without VFb |
|---|---|---|---|---|---|---|---|
Steroid-treated diseases | |||||||
Leukemia | •Halton (STOPP) (2009) •Prospective, observational | •Age from 1 month to 17 yrs with ALL •Bone health assessment initiated within 30 days of chemotherapy initiation | •N = 186 •Age: median (IQR) = 5.3 (3.4, 9.7) at chemotherapy initiation •Male: 58 % | •Assessed at baseline and then annually for every patient | •Lateral spine X-ray •Genant method | •Prevalence: 16 % •Time: within 30 days of chemotherapy initiation | Multivariable model: •Back pain: OR = 4.7 (1.5, 14.5) •↓Second metacarpal percent cortical area Z-score: OR = 2.0 (1.0, 3.2) •↓LSBMD Z-score: OR = 1.8 (1.1, 2.9) |
Leukemia | •Alos (STOPP) (2012) •Prospective, observational | •Age from 1 month to 17 yrs with ALL •Bone health assessment initiated within 30 days of chemotherapy initiation | •N = 155 •Age: median (min, max) = 6.4 (2.2, 18.0) at 12 months after chemotherapy initiation •Male: 59 % | •Assessed at baseline and then annually for every patient | •Lateral spine X-ray •Genant method | •Incidence: 16 % •Time: 12 months after chemotherapy initiation | Multivariable models: •Prevalent VF (yes vs. no): OR = 7.30 (2.30, 23.14) •↓LSBMD Z-score: OR = 1.8 (1.2, 2.7) •Prevalent VF (mild vs. none): OR = 7.6 (1.8, 31.8) •Prevalent VF (moderate/severe vs. none): OR = 7.0 (1.6, 30.2) |
Leukemia | •Cummings (STOPP) (2015) •Prospective, observational | •Age from 1 month to 17 yrs with ALL •Bone health assessment initiated within 30 days of chemotherapy initiation | •N = 186 •Age: median (IQR) = 5.3 (3.4, 9.7) at chemotherapy initiation •Male: 58 % | •Assessed at baseline and then annually for every patient | •Lateral spine X-ray •Genant method | •Incidence: 8.7 per 100 person-years •Cumulative incidence: 26.4 % •Time: over 4 years after chemotherapy initiation | Multivariable models: •Prevalent VF (mild vs. none): HR = 4.2 (1.9, 9.6) •Prevalent VF (moderate/severe vs. none): HR = 6.2 (3.4, 11.4) •↑Average daily GC (10 mg/m2): HR = 5.9 (3.0, 11.8) •↓ LSBMD Z-score at the time of VF assessment: HR = 1.6 (1.2, 2.2) •↓age: HR = 1.1 (1.0, 2.2) •↑recent (12 months preceding VF assessment) average daily GC (10 mg/m2): HR = 5.1 (2.8, 9.5) •↑Recent GC dose intensity (10 mg/m2): HR = 1.2 (1.1, 1.4) |
Rheumatic diseases | •Huber (STOPP) (2010) •Prospective, observational | •Age from 1 month to 17 yrs •Within 30 days of first-time GC therapy for the treatment of underlying rheumatic diseases including JDM, JIA (excluding systemic arthritis), SLE, JIA (systemic arthritis), SV, and others | •N = 134 •Age: median (min, max) = 10.0 (1.4, 16.9) •Male: 35 % | •Assessed at baseline and then annually for every patient | •Lateral spine X-ray •Genant method | •Prevalence: 7 % •Time: within 30 days of first-time GC therapy | Univariate model: •Back pain: OR = 10.6 (2.1, 53.8) |
Juvenile arthritis | •Markula-Patjas (2012) •Cross-sectional | •Age <19 yrs •Polyarticular JIA for ≥5 years, or systemic arthritis for ≥3 yrs | •N = 50 •Age: median (range) = 14.8 (7.0, 18.7) •Male: 18 % | •Assessed following enrollment into the study | •Lateral spine X-ray •Method proposed by Makitiec | •Prevalence: 22 % •Time: after enrollment into the study | Univariate model: •Cumulative GC dose >75 mg/kg: OR = 7.2 (1.37, 38.0) •Disease activity (CHAQ > 0.5): OR = 7.2 (1.58, 32.9) •BMI Z-score > 2: OR = 4.7 (1.13, 19.21) |
Juvenile arthritis | •Varonos (1987) •Retrospective, case-control | Group with VF: •Age < 16 yrs •With juvenile chronic arthritis treated with GC •Developed at least one vertebral collapse Group without VF: •With juvenile chronic arthritis •Without fractures •Received GC for at least 1 year •At some time point in time had mean daily dose at least 5 mg of prednisolone | Group with VF: •N = 23 •Age: mean (min, max) = 4.8 (1.3, 9.9) •Male: % not reported Group without VF: •N = 23 •Age: mean (min, max) = 3.8 (0.9, 11.9) •Male: % not reported | •Routinely performed annually from beginning of GC for every patient •More frequently if chest pain or loss of height occurred | •Lateral spine X-ray •Method proposed by Jensen and Tougaardd | N/A | Statistical test for comparing children with and without VF: •↑Average daily dose of GC •↑ Weight-adjusted average daily dose of GC |
Rheumatic diseases | •Nakhla (2009) •Cross-sectional | •≤18 yrs •Had earlier or current exposure to methotrexate, corticosteroids, or both •Had the following diagnosis: JIA, CTD (including SLE, JDM, and SV) | •N = 90 •Age: median (min, max) = 13.1 (4.3, 18.0) •Male: 24 % | •Assessed after enrollment into the study for every patient | •Lateral spine X-ray •Genant method | •Prevalence: 19 % •Time: after enrollment into the study | Multivariable models (predictors of the number of VF events): •Sex (Male vs. Female): OR = 6.04 (2.85, 12.81) •↑Cumulative GC (g/kg): OR = 4.50 (1.42, 14.28) •↑BMI Z-score: OR = 1.49 (1.05, 2.09) |
Rheumatic diseases | •Rodd (STOPP) (2012) •Prospective, observational | •Age from 1 month to 17 yrs •Within 30 days of first-time GC therapy for the treatment of underlying rheumatic diseases including JDM, JIA (excluding systemic arthritis), SLE, JIA (systemic arthritis), SV, and others | •N = 117 •Age: median (min, max) = 11.0 (2.3, 17.9) at 12 months after GC initiation •Male: 37 % | •Assessed at baseline and then annually for every patient | •Lateral spine X-ray •Genant method | •Incidence: 5 % •Time: 12 months after GC initiation | Statistical test for comparing children with and without VF: •↑BMI Z-score, study entry to 6 months •↑Weight Z-score, study entry to 6 months •↓LSBMD Z-score, study entry to 6 months •LSBMD Z-score <−2.0 at 12 months •↑Cumulative GC •↑Average daily GC |
Rheumatic diseases | •LeBlanc (2015) •Prospective, observational | •Age from 1 month to 17 yrs •Within 30 days of first-time GC therapy for the treatment of underlying rheumatic diseases including JDM, JIA (excluding systemic arthritis), SLE, JIA (systemic arthritis), SV, and others | •N = 134 •Age: mean (SD) = 9.9 (4.4) at the time of GC initiation •Male: 35 % | •Assessed at baseline and then annually for every patient | •Lateral spine X-ray •Genant method | •Incidence: 4.4 per 100 person-years •Cumulative incidence: 12.4 % •Time: over 3 year after GC initiation | Multivariable models: •↑Average daily GC dose (0.5 mg/kg): HR = 2.0 (1.1, 3.5) •↑VAS score, baseline to 12 months: HR = 1.4 (1.1, 1.7) •↑BMI Z-score in the first 6 months preceding each annual VF assessment: HR = 3.2 (1.6, 6.5) •↓LSBMD Z-score, baseline to 6 months: HR = 3.0 (1.1, 8.1)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




