If the nutritional index is equal to zero or negative, oral or parenteral hyperalimentation must be given. If a patient loses more than 5 kg of weight before an elective surgery, a kind of supportive therapy must be considered. In trauma or infected cases, the basal metabolism rate can increase up to 30–50%, also 0.5C0 increase in body temperature increases the basal metabolism rate up to 12%. Alimentation must be adjusted in these situations. Malnutrition affects humoral and cellular immune response negatively. In malnutrition, chemotaxis of polymorphonuclear leukocytes, bacterial phagocytosis, and serum complement function deteriorate [18].
In the prevention of infection, both cellular and humoral immune system is very important. Also reticuloendothelial system organs like liver, spleen, and lymph nodes are important in the prevention of infection. If there is a defect in anyone of these, then opportunist microorganisms can produce infection. This defect can be congenital or acquired. Pseudomonas in heroin addicts, Salmonella and S. aureus in sickle cell anemia are the most seen organisms. Diabetes mellitus, alcoholism, hematological malignancies, and cytotoxic treatments cause neutrophil function defect and S. aureus, gram negative bacillus, aspergillus, and candida infections are mostly seen because of these defects. Immunoglobulins and complement systems are the important components of the humoral immunity. In hypogammaglobulinemic or post-splenectomy patients, encapsulated bacteria (Pneumococcus, Haemophilus influenza, Streptococcus, Neisseria) are commonly seen as infectious agents.
Lymphoma, systemic lupus erythematosus (SLE), steroid treatment, and malnutrition impair the cellular mechanism and cause mycobacterium, fungal, and herpes infections. Chronic granulomatous disease, hemophilia, hypogammaglobulinemia, sickle cell anemia, terminal complement pathway deficiency, and leucocyte adhesion disorders are the congenital disorders that increase the risk of infection. Acquired diseases are diabetes mellitus, rheumatoid arthritis, hematological malignancies, drugs that depress immune system, transplantation, collagen vascular diseases, uremia, malnutrition, and radiation therapy. The risk of infection in total joint replacement increases six times in diabetes mellitus and rheumatoid arthritis patients.
Host Response
The host responses to bacterial invasion with inflammatory reaction, first. Acute inflammatory reaction is a protective response that causes cell damage and also removes these residual necrotic tissues and cells. Also, the act that is named as repairing, which means the fulfilling of defect with fibrous scar tissue, and regeneration of parenchymal cells of damaged tissue, occurs concomitantly [19–21].
First and Congenital Immune Response
Either tissue damage or the bacteria itself activates the complement system. Complement activation causes vasodilatation, edema, and polymorphonuclear leucocyte migration. Polymorphonuclear leucocytes invade the bacteria by opsonin and provide a suitable environment for phagocytosis. Interleukin-1, interleukin-6, and tumor necrosis factor released by damaged tissue provide more polymorphonuclear cells and macrophage migration to the area. Activated phagocytic cells produce free oxygen radicals, which is essential for host immune response. Pain, swelling, erythema, and warmth are clinical evidences of vasodilatation and tissue edema.
Acquired Immune Response
T-lymphocytes are responsible for cellular immune response and B-lymphocytes for humoral. In acute inflammation, polymorphonuclear cells are predominant, whereas mononuclear cells (lymphocyte, macrophage, plasma cells) are predominant in chronic inflammation. Chronic inflammation is a prolonged inflammation in which acute inflammation, cell damage, and recovery progresses together.
If the infection is septic arthritis, bacteria damages the cartilage tissue by acting on glycosaminoglycan, which is a subunit of proteoglycan molecule. Glycosaminoglycan, which consists of chondroitin sulfate, keratan sulfate, dermatan sulfate, and hyaluronic acid, is a macromolecule that increases physical property of cartilage tissue, maintains retention of fluid, and provides strength to pressure. Damage to glycosaminoglycan causes cartilage destruction and collagen break and also secondary osteoarthritis.
Biofilm
Another virulence factor that is efficient in infections following orthopedic operations is the binding of the organism to biomaterials and organic tissues and its capacity to form biofilm. Biofilm is a gel-like polysaccharide-containing layer that is formed by the binding of bacteria to each other or to the materials. Biofilm structure is a barrier that blocks the host’s immune system elements and the antibiotics to reach the organisms [57]. Eighty percent of biofilms found in a body are formed by Staphylococci. The reason is that these microorganisms are found in skin flora and they can accumulate along the catheters easily, and they can easily reach the deep tissues and the implants during the manipulations (Fig. 30.1).
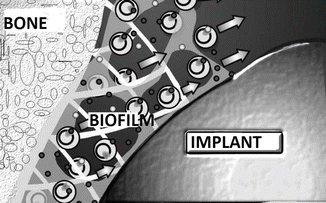

Fig. 30.1
Biofilm
Biofilms may be found on catheters, implants, heart valves, contact lenses, kidney stones, and organic tissues. All foreign bodies are recognized by the body and covered with a protein layer containing laminin, fibronectin, vitronectin, collagen, and fibrinogen. This structure called slime factor can bind the microorganism to both the implant and the organic tissues [58]. In addition, a group of protein called “microbial surface components recognizing adhesive matrix molecules” (MSCRAMM) on the surface of the Staphylococci makes a tight connection with this slime factor and forms glycocalyx (biofilm). Teichoic acid also plays a major role in this connection [59]. The main material of the biofilm layer is N-Acetylglucosamine and it forms two different polysaccharide structures. Type 2 polysaccharide structure is responsible for the intercellular aggregation and is alternatively called polysaccharide intercellular adhesin (PIA). This structure is the component that has the ability to bind to the hydrophilic surfaces with physicochemical bonds [60]. To have knowledge about these molecules in Staphylococcus infections is important to develop new treatment modalities, because these structures may provide protective immunity when they are injected to individuals in their purified form (Staphylococcus vaccine) [61]. These researches are the basis of the policy for the development of vaccines against the nosocomial infections that may develop in patients that will stay for a long period at the hospitals.
The adhesion on implant generally happens in two steps. The first step is the holding of the microorganism on the surface with physicochemical gravity, such as hydrophobic forces and van der Waals forces (adhesion phase). The second step is the development of slime factor with special protein structures, and the continued accumulation of the bacteria (accumulation phase) [62]. Every step requires different molecules. The efficient structure in both periods is the “biofilm associated protein.”
Biofilm formation is seen in infections where the implant is used. The implant is isolated and covered with a layer formed by macromolecules, then microorganisms attach to implant and to this layer. Glycocalyx formation occurs when the exopolymers cover the film layer that is formed by the macromolecules. Bacteria within this glycocalyx layer are protected from antibiotics, humoral and cellular immune system elements. Inside the biofilm layer there is not enough metabolite and waste products formed by bacteria also accumulate; therefore bacterial proliferation is decreased or sometimes proliferation does not occur. Bacteria inside the biofilm transform into an organized cell population and become structurally and functionally different. Genetic differentiation occurs with cell to cell signaling. Because of the after-mentioned reasons, in order to eradicate infection from infected implants, the biofilm layer and the implant must be removed from the area of infection. It should not be forgotten that the ability of Staphylococcus to form a biofilm is high.
Implant-related infections can occur by direct contamination during surgery or by hematogenous spread from a remote infection. Also infection can occur by direct or lymphatic spread from a nearby infection focus. Pathogenesis and duration of development of infection after arthroplasty vary according to the pathogenic microorganism. Therefore, prosthesis infections can be classified in three different categories:
(a)
Acute infection
(b)
Subacute infection
(c)
Chronic infection
This classification also determines the approach to treatment. Early infection after surgery is defined as the emergence of signs of infection at the implant site within the first 3 months. Direct contamination during surgery is the reason for early infection and virulent microorganisms are mainly the causative agent (e.g., S. aureus). Intractable local pain, erythema, hematoma, and fever are the clinical findings. Subacute infection is seen 3–24 months after surgery. Mostly low virulence of microorganisms (coagulase-negative Staphylococci, Propionibacterium acnes) is the causative agent. Continuous or increasing pain and early loosening is seen; sometimes it is very difficult to discriminate from aseptic loosening, because signs and symptoms related to infection may not be seen. Chronic infection is defined as infection seen 2 years or more after surgery. Infectious agents in this period cause infection by hematogenous spread from teeth, skin, respiratory tract, and urinary tract infections. Hematogenous infection of the internal fixation devices is more rare. Similarly, in the early period (within 10 weeks) most commonly seen causative agents were S. aureus and gram (−) bacilli, while in the later period (after 10 weeks) low virulence microorganisms like coagulase negative Staphylococci are the causative agents [22–26].
Infection Prophylaxis
Antimicrobial prophylaxis is the most effective way to decrease infection prevalence after orthopedic surgery [27, 28]. First or second generation cephalosporins like cefazolin, cefamandole, or cefuroxime are used in orthopedic surgery. If the patient has an allergy to these drugs or if the hospital has a high prevalence of methicillin-resistant S. aureus infection then vancomycin or teicoplanin is used. In order to ensure an effective prophylaxis, a minimum inhibitory concentration level should be achieved during surgery. Risk of surgical site infection increases six times when prophylaxis was done 2 h prior to surgery or 3 h after surgery. Studies demonstrate that in order to obtain the most effective tissue concentration, antibiotic prophylaxis must be done 30–60 min prior to surgery or tourniquet application. Clindamycin may be used in the presence of penicillin allergy. An additional dose should be given every 4 h or when blood loss is more than 1,000 ml during surgery. Prophylaxis should not be given more than 24 h even in the presence of catheters and drains. Complications like drug-related fever, allergic reactions, thrombophlebitis, and superinfections can be seen if used more than 24 h. Risk of bacteremia has shown to increase in intensive care unit patients when antibiotics were used more than 4 days. In the presence of open fracture or during an elective surgery, irrigation with liters of serum physiologic is a very effective way to decrease the number of contaminated bacteria and so is an important step in infection prophylaxis. Irrigation during surgery also prevents tissue desiccation and lessens tissue necrosis. In Gustilo Anderson grade I and II open fractures, prophylactic treatment with cefuroxime or cefamandole for 1 day and in grade III open fracture, a preemptive treatment with an antistaphylococcal drug like i.v. amoxicillin/clavulanic acid or cefuroxime for 5–7 days is usually enough.
Most of the infections in orthopedic surgery are coagulase (−) S. aureus and S. epidermidis, which are originated from skin flora. Factors that can be managed by a surgeon in the prevention of infections are very important. The source of an infectious agent can either be patient or surgeon’s skin flora. Skin hygiene is more important in orthopedic surgery than other surgical departments. It is impossible to sterilize the skin but it can be possible to minimize bacterial burden. One of the most important factors in the development of infection is the number of bacteria. Skin and hairy tissues are cleared by hexachlorophene, chlorhexidine, alcohol, or iodine. However, hairy follicles cannot be cleared as well and as time expands bacteria proliferates during surgery and the risk of contamination increases. Use of iodine-based surgical drapes also prevents the contamination of bacteria from hair follicles. In the past, disinfectants that can penetrate to hair follicles, sebaceous and sweat glands were used, but it must not be forgotten that absorption of these disinfectants lead to neurotoxicity. Bristles must be removed in the operating room just before the surgery. Removal of the bristles one day prior to surgery leads to microtrauma to skin, and bacterial colonization of this traumatized areas occur. Removal of bristles must be done with using clippers not with razor blades or other sharp objects.
As mentioned above, another source of microorganism contamination is the skin flora of the surgical team. Laceration of the gloves of the surgeon is seen to occur in 48 % of the cases in orthopedic surgery. Glove laceration is most commonly seen in the first and second fingers of the nondominant arm. It is appropriate to use double gloves and the usage of gloves that has an indicator to laceration is also helpful. Appropriate hand washing before surgery and usage of masks and caps that cover the whole face except the eyes is important in the prevention of contamination (Fig. 30.2). The usage of a large safety glasses also decreases contamination and protects the surgeon’s conjunctiva from contamination with patient’s blood and body fluids. “Body exhaust systems” can be used in order to decrease contamination (Fig. 30.3). Bacteria suspended in the air of the operating room is another source for bacterial contamination. Staff in the operating room is the source of these bacteria. A person can release between 5,000 and 55,000 particles/min. There are 7,000 particles per liter of air in a conventional operating room theatre. This number and hence the contamination risk can be decreased significantly by the usage of HEPA filters, positive pressured theaters, and laminar flow. The air pressure in the operating room must be higher than in the corridors. Filtered clean air from HEPA filters must enter the operating room, then with the effect of positive pressure air travel from the operating room to corridors and must leave the operating theatre from the main entrance. For an effective system, windows in the operating rooms should not be openable, and an airlock system at the entrance of the operating theatre is required. A decreasing percentage of the surface of staff’s skin that has contact with the air also lessens the amount of particles. Operating rooms must be 19 °C, corridors 21 °C, and resting rooms 23 °C. In past, UV light has been used to diminish the amount of bacteria but because of the adverse effects and its time-consuming effect, it is not recommended recently.

Fig. 30.2
Appropriate mask and cap usage to decrease contamination and particle release

Fig. 30.3
Picture of a surgical team wearing body exhaust system
Clinical Characteristics and Diagnosis of Osteomyelitis
Hematogenous osteomyelitis refers to a bone infection that is transported by blood from the region far away to the infectious area. That kind of osteomyelitis occurs mostly in children and sometimes after total joint arthroplasty operations. In children with incomplete skeletal maturation, because of decreased blood flow in the metaphyseal region, bacteria settle in this region. In some locations (e.g., proximal femur) the metaphyseal part of the bone is inside the joint so osteomyelitis in these regions can easily complicate with septic arthritis. Because of the avascular nature of the joint, drainage of the joint must be done in order to treat joint infections. Pathogenic microorganisms vary according to the age of the child. In neonates and infants staphylococcus, between 6 months and 3 years old H. influenza, after 3 years old staphylococcus, and in adolescents staphylococcus species are the most commonly seen causative agents (Tables 30.1, 30.2, 30.3, and 30.4).
Table 30.1
Most common causative agents for osteomyelitis according to age
Neonate and infant |
|---|
Staphylococci |
Streptococci |
Gram (−) bacteria |
Table 30.2
Most common causative agents for osteomyelitis according to age
6 months to 3 years old |
Haemophilus influenzae Type B |
Staphylococci |
Streptococci |
Table 30.3
Most common causative agents for osteomyelitis according to age
3 years old–Adolescent |
Staphylococci |
Streptococci |
Table 30.4
Most common causative agents for osteomyelitis according to age
Adolescent |
Staphylococci |
Streptococci |
Neisseria gonorrhoeae (rarely) |
Joint aspiration, culture, and direct microscopy are very important tools for the diagnosis. Especially for septic arthritis 50,000/mm3 or more leucocytes in direct microscopy is diagnostic. Local edema, warmth, redness, sensitivity, limitation of joint motion, and systemic fever can be clinical findings. Periosteal reaction, bone destruction, deterioration of trabecules, and increase in soft tissue density can be seen in direct radiological examination. Complete blood count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) are used both for the diagnosis and follow-up. CRP can elevate within hours in acute infection and can reach very high values within 48 h. After recovery of infection, CRP falls to normal values within weeks. CRP is a more precise diagnostic tool both for diagnosis and follow-up. ESR needs longer time to reach normal values.
In the first 2 weeks, direct radiological examination findings may be less clear, so bone scintigraphy can be useful for the diagnosis of septic arthritis and osteomyelitis. Imaging with 99mTc-methylene diphosphonate (99mTc-MDP) is the initial method of choice. Scintigraphy shows osteoblastic activity and blood flow. The third phase is the phase that is 3 h after injection and shows bone involvement. Scintigraphy is a sensitive test in the diagnosis of septic arthritis and osteomyelitis. But in newborns, we should be aware of false (−) results. On the other side, in pre-existing chronic diseases of bone, especially in sickle cell anemia or bony metastases of tumors, we can see false (+) results. In the healing stage of bone fracture, increased uptake can be seen too. Ga 67(gallium citrate) can be used in musculoskeletal scintigraphic examinations. But it can take 2 days for results and it is time consuming. Using radioactive indium labelled leucocytes is rigorous and needs a long time.
CT and MRI are useful methods in the diagnosis and treatment of osteomyelitis. CT helps in surgical planning by imaging cortical bone and sequestrum. MRI is a more sensitive and specific method and helpful in surgical planning. It shows abscess in sinuses. It is not an expensive method anymore and there is no radiation exposure. However, it is not useful for cases where implants were used [29–31].
Treatment
After the diagnosis of septic arthritis or osteomyelitis, cultures from blood and deep soft tissues are taken; then drainage and debridement of the infected and necrotic tissues is planned immediately. In order to treat infection, all necrotic tissues must be removed. Necrotic tissue is a suitable environment for bacteria. Also host defense mechanisms try to remove necrotic material. This causes continuous drainage from sinus tracts. Sometimes a sequestered fragment is removed from sinus opening after months.
Medical treatment without surgery can be done in children with acute osteomyelitis if it is diagnosed early. In order to be successful, diagnosis must be done early and antibiotics specific to the pathogenic microorganism must be given. In the treatment of acute osteomyelitis and septic arthritis, parenteral antibiotics are administered for the first 1–2 weeks followed by 4–5 weeks of oral antibiotics. However, the time of antibiotic administration must be longer in diabetics or in other immunocompromised patients. Response to treatment is followed with both clinical and laboratory findings. ESR and CRP levels must be followed during treatment, and if regression of the ESR and CRP levels is not seen, then antibiotics must be given for longer periods. Repeated surgical debridement of necrotic materials must be done in patients in whom improvement cannot be seen. It must be known that in order to control and treat implant-related infections, implants like plate, screw, prosthesis, or bone cement must be removed. In the presence of fracture, implant may be retained with antibiotic suppression until fracture healing occurs.
In the treatment of chronic infections with large necrotic soft tissues, rotational or free flaps, myocutaneous or fasciocutaneous composite greft usage can be successful after a radical debridement. These kinds of flaps and grefts fill the death spaces and also carry living tissue to infection areas thus increasing perfusion and helping fracture healing.
All necrotic bone must be removed. The bony defect formed after debridement can be filled with autogrefts or allogrefts. In large defects, distraction osteogenesis and filling the defect with segment transport can be done.
Local antibiotic treatments and vectors were developed to provide the antibiotics to reach to high concentration levels especially at the infection area, where systemic applications may cause toxic effects. These vectors are bone cement, synthetic and organic bone grafts, plaster of Paris, polylactate and polyglycolate membranes, Ca sulfate-phosphate, hydroxyapatite, and amylose-starch implants [63–68].
The use of the most frequent application method, methacrylate beads containing gentamicin, both provides to fill the dead areas and to keep the local antibiotic concentration at high levels [69]. With this method, it was shown that the local antibiotic concentration is 200 times higher than with systemic application [70]. Biosoluble vector systems are more advantageous, because they do not need to be extracted later.
Infection and Total Joint Replacement
Nowadays, infection rate after total joint replacement surgery is less than 1 % but it is still the most serious complication after arthroplasty. Infection can present like acute hematogenous osteomyelitis, chronic osteomyelitis, or septic arthritis [32].
Resting pain is the most frequent finding. Normally pain around the surgical wound diminishes within days. Continuous or even increasing pain after surgery is an important finding. Fever, chills, purulent drainage, and sinus formation are rarely seen after periprosthetic infections. Erythema around incision can be seen after total knee arthroplasty and it is not specific to infection. If other findings accompany erythema like pain, close follow-up for infection is necessary. Blood sample for complete blood count, ESR, and CRP are taken from the patient. These laboratory examinations are helpful for the diagnosis, but gram stain, direct microscopy, and the culture of the tissue specimen that are taken by surgery or aspiration are exact diagnostic methods. Aspiration of synovial fluid is a simple, quick, and definitive method. More than 1,700 leucocytes in 1 mm3 of fluid and neutrophils >65 % is a potent indicator of infection. And these numbers are very much lower than the numbers used in the diagnosis of septic arthritis. Sensitivity of this method is 94 % and 97 % and specificity is 88 % and 98 %, respectively. In frozen sections of specimens that are taken during surgery, the number of polymorphonuclear leucocytes seen in every field (at big magnification) is informative about infection. Polymorphonuclear leucocyte count in every field >5 increases the possibility of infection; >10 is a strong indicator of infection. Tissue specimens should not be taken from the region close to fracture, because it causes false (+) results. It must be taken from the granulation tissue close to the components of prosthesis (Fig. 30.4). If the implant has been removed, the sonication method that separates microorganisms apart the surface of the implant and subsequent culturing increases the sensitivity of culture. This method is gaining popularity nowadays.
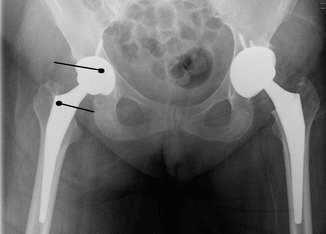 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








