Fig. 2.1
Aseptic loosening might be explained by four different concepts: wear particle disease, interface sealing effect, hydrostatic fluid pressure concept, bacterial endotoxin theory
Aseptic loosening causal concepts:
1.
Wear particle disease
2.
Interface sealing effect
3.
Hydrostatic fluid pressure concept
4.
Bacterial endotoxin theory
2.2 Wear Particle Disease
Wear particles are released in every TKR [9, 16, 18, 19]. The amount, type and size of wear particles depend on the bearing materials, the orientation and the position of the TKR [9, 16, 18, 19]. The wear debris and the bone cement interact with macrophages, which then secrete proinflammatory mediators and proteolytic enzymes, resulting in an increased bone resorption [9, 16–18].
Amount, type and size of wear particles depend on bearing materials, orientation and position of the TKR.
Wear particles from polyethylene, metal or ceramic bearings result in a local chronic, granulomatous inflammatory response, which finally results in the formation of a pseudomembrane at the bone-cement-prosthesis interface [9, 16, 19]. The main components of such a pseudomembrane are macrophages and giant foreign body cells incorporating different types of wear particles [9, 16– 18]. As precursor cells, the macrophages could differentiate into bone-resorbing osteoclasts [9, 16–18]. Fibroblasts, osteoblasts, lymphocytes and polymorphonuclear leucocytes were also found [16–18].
The main components of a periprosthetic pseudomembrane are macrophages and giant foreign body cells, which incorporate wear particles.
The complex inflammatory process is medi- ated by numerous proinflammatory and anti- inflammatory cytokines, chemokines and several other mediators [9, 17].
It is the consensus that wear particle size and shape, the amount of wear and the wear material itself, whether polyethylene, metal or ceramic, directly influence the changes in the balance of bone resorption and bone formation [9, 16, 20]. The characteristics of wear debris are dependent on a variety of factors such as the type of wear, the implanted TKR, the cementing technique, the patient and finally the operating technique [9, 18]. It has been shown that larger-sized particles (>1 cm) as well as very small wear particles cannot be phagocytised by macrophages [9, 18].
Larger-sized particles (>1 cm) as well as very small particles cannot be phagocytised by macrophages. These can only incorporate particles of 0.3–10 μm size.
Only particles within the range of 0.3–10.0 μm are able to activate macrophages, which then lead to an increase of proinflammatory and osteolytic cytokines [9, 18]. While larger particles, >5 μm in diameter, are phagocytised by osteoclasts, smaller particles, <2 μm in diameter, are taken up by macrophages [9, 18].
For a closer look at the process of aseptic loosening, we need to focus on the place where the process is actually happening: the bone-cement-prosthesis interface, the periprosthetic membrane and the synovial membrane.
2.3 Role of Interface Sealing and Hydrostatic Fluid Pressure
Sealing off the bone-cement-prosthesis interface has been identified as an important factor to improve longevity of orthopaedic prostheses [9, 21–26]. It is believed that sealing off the interface impairs wear particles, synovial fluid and macrophages from being pumped into this interface [21]. Thus, bone resorption in this prosthetic interface might be prevented. It is also clear that a well-functioning cement mantle as well as a circumferentially porous- or hydroxyapatite-coated, osseointegrated implant prevents the migration of cells [11]. However, it seems unlikely that sealed interfaces could permanently withstand excessive wear debris (Fig. 2.2).
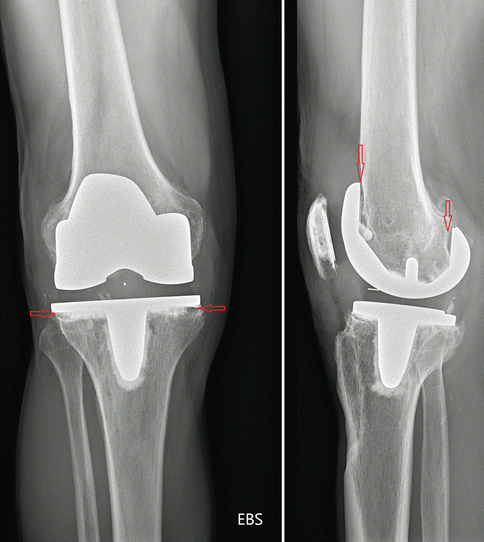

Fig. 2.2
Aseptic loosening due to insufficient sealing of bone-prosthesis interface by cement
Sealing off the bone-cement-prosthesis interface impairs wear particles from intruding into it and delaying aseptic loosening.
Another possible genesis of aseptic loosening is increased hydrostatic pressure that is due to an increased amount of synovial fluid [22–26]. Under these circumstances, synovial fluid is pumped into the interface. It has been shown in animal studies that this increased pressure then triggers macrophages to produce “osteolytic” cytokines [22–26]. It seems that all these causal concepts do not really represent separate mechanisms of aseptic loosening, but act synergistically and influence each other.
Hydrostatic pressure theory—an increased amount of synovial fluid leads to pumping of wear particles into the bone-prosthesis interface.
2.4 Bacterial Endotoxins as a Cause of Aseptic Loosening
A few authors have recently highlighted the possibility of bacterial endotoxins as a cause of aseptic loosening [16]. According to their studies it could be that “aseptic” loosening of implants cannot be separated from an infection [16].
Bacterial endotoxins as well as bacterial remnants are considered to be responsible for a chronic activation of the macrophage system leading to (aseptic) loosening.
Mariani et al. investigated 50 patients with signs of joint infection and reported that 32 of these cultures of synovial fluid were only positive in polymerase chain reaction, while only 15 showed positive conventional cultures. This difference in diagnostic sensitivity was explained by the fact that 13 of the PCR-positive but culture-negative samples showed hardly any level of infection [27]. This phenomenon is either caused by remnants of bacteria such as the bacterial membrane or endotoxins, which are left even when the bacteria have been neutralised by antibiotics or immune cells, or by bacterial biofilms, which allow bacteria to even survive antibiotic treatments [16]. Due to their low biological activity, bacterial remnants and endotoxins are difficult to culture [16]. Bacterial biofilms can develop on implant surfaces at the time of primary surgery or at a later stage hematogenously [16]. A major problem is that the biofilm protects bacteria against antibiotics and immune cells and the immune system is not capable of successfully attacking these [16].
In contrast, bacterial remnants, endotoxins and bacterial biofilms present antigens to immune cells such as macrophages, which then continuously produce proteolytic enzymes and cytokines and initiate a permanent inflammatory response, finally leading to osteolyses around the TKR [16].
2.5 Bone-Cement-Prosthesis Interface
The bone-cement-prosthesis interface is the place where aseptic loosening is initiated.
Implantation of a TKR always leads to remodelling of the bone as a result of the new loading conditions. During the process of remodelling, a periprosthetic membrane (Fig. 2.3) between the prosthesis and the adjacent bone is established, which predominantly consists of macrophages, fibroblasts, multinuclear giant cells, such as osteoclasts, and lymphocytes [9, 18, 19, 28]. This periprosthetic membrane later initiates the aseptic loosening process [9, 19].
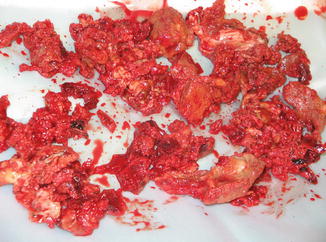

Fig. 2.3
Macroscopic image of a periprosthetic membrane
Morawietz et al. have proposed a classification system for the periprosthetic membrane from loosened total hip and knee prostheses [28]. Based on histomorphological criteria and polarised light microscopy, they identified four distinct types of periprosthetic membranes in a series of 268 patients: periprosthetic membrane of wear particle type (type I), periprosthetic membrane of infectious type (type II), periprosthetic membrane of combined type (type III) and periprosthetic membrane of indifferent type (type IV).
The wear particle type (I) was predominantly characterised by macrophages and multinuclear giant cells and different types of abundant wear debris. Only a few lymphocytes were found. The periprosthetic membrane of the infectious type (type II) can be subdivided into the low-grade infection and the acute fulminant infection. The low-grade infection as a chronic granulomatous inflammation is characterised by active fibroblasts, neutrophilic granulocytes, numerous plasma cells and few lymphocytes.
The periprosthetic membrane of the combined type (type III) is a combination of types I and II. The periprosthetic membrane of the indifferent type (type IV) consists of collagen-rich fibrous tissue. Only few cells, mostly fibroblasts and macrophages, cover the surface of the periprosthetic membrane.
A variety of different cytokines, chemokines and growth factors have been identified in the periprosthetic membrane surrounding failed TKR (Table 2.1) [9, 18, 19].
El-Warrak et al. studied the process of aseptic loosening within the interface membrane in cemented total hip arthroplasty using an experimental sheep model [45]. One group receiving a complete cement mantle served as control; in the other group a standardised, lateral, primary defect in the cement mantle was established [45]. A distinct interface membrane was found in both groups, but in the control group the interface membrane was thinner and biologically less active [45].
In another landmark prospective study, Goodman et al. characterised the cell composition of the periprosthetic interface in cemented (n = 65) and uncemented (n = 36) total knee (n = 16) and hip arthroplasty (n = 85) undergoing revision surgery. The major cell types and cytokines found were investigated using immunohistochemistry and in situ hybridization [11] (Table 2.2).
Loose versus stable | Cemented versus uncemented | With versus without osteolysis | Tissue characteristics | Histology |
|---|---|---|---|---|
Loose | Cemented | With osteolysis | Extensive yellow-brown fibrotic tissue around prosthesis osteolyses extends deeply into the prosthesis adjacent bone | Highly vascularised fibrous tissue |
Synovial-like lining layer adjacent to cement layer | ||||
Macrophages with phagocytised cement, polyethylene and metal particles | ||||
Under polarised light a bluish-yellow tissue birefringence was seen in highly cellular areas, which reflected submicron polyethylene particles within the tissue | ||||
Greenish-grey bone-cement remnants were surrounded by macrophages | ||||
Lymphocytes rarely found throughout the tissue | ||||
Without osteolysis | Prosthesis surrounded by a more organised fibrous tissue | Variable number of cells and wear debris | ||
Volume much less than that found around loose implants with osteolysis | Some areas with granuloma-like tissue but in others macrophages rarely found | |||
Synovial-like lining occasionally | ||||
Well fixed | Cemented without radiological evidence of loosening | Little fibrous tissue around prosthesis | Scattered macrophages, lymphocytes and wear debris | |
Often tightly adherent to the prosthesis or underlying bone | No synovial-like lining layer | |||
Loose, cementless with osteolysis | Less plentiful, more fibrous and less cellular than in loose cemented group | Bluish-yellow tissue birefringence of polyethylene particles | ||
Phagocytosed polyethylene and metal debris | ||||
Areas of black-coloured tissue containing large amounts of phagocytosed and interstitial metal debris | ||||
Loose, cementless without osteolysis | Prosthesis surrounded by a more organised fibrous tissue | Variable number of cells and wear debris | ||
Volume much less than that found around loose implants with osteolysis | Some areas with granuloma-like tissue but in others macrophages rarely found | |||
Synovial-like lining occasionally | ||||
Well fixed, cementless without radiological evidence of loosening
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





