Biological Responses to Metal Debris and Metal Ions
Patricia A. Campbell and Karren Takamura
Key Points
Introduction
Metal-on-metal (MOM) bearings were reintroduced in the late 1980s with the goal of reducing polyethylene (PE)-induced osteolysis and aseptic loosening, thus improving implant longevity, particularly in young and active patients. MOM articulations produce significantly less volumetric wear compared with metal-on-polyethylene bearings, but the particles are much smaller (nanometer-sized) and much more numerous. These wear products have the potential to cause locally aggressive biological responses that may be a major limiting factor to the long-term success of MOM implants. Biological reactions to metal particles and ionic corrosion products are the focus of this chapter.
Basic Science of Wear Debris
Polyethylene Wear and Osteolysis
It is useful to discuss metal wear debris reactivity from the context of what has been learned from studies of PE debris. Debate is ongoing as to which factor is most important for the cellular response to wear particles, but it is generally accepted that for PE particles, their submicron to micron size, the nondegradable nature of the polymer, and the high volumes that are typically produced are the characteristics that, when combined, lead to an aggressive local response that results in bone resorption and implant loosening.1–6 The more subtle particle characteristics, such as aspect ratio, surface roughness, and the composition of absorbed proteins, may also determine bioreactivity, but these are less well understood.2 Much work on the bioreactivity of wear particles has been performed using particles of commercial polymers, including the powder form of ultra-high-molecular-weight polyethylene that is used as the base material for fabrication of bearing inserts for hip or knee implants. In these studies, this material was chosen to simulate the debris produced by metal-polyethylene hip replacements, because the primary goal of these studies was to understand and thus prevent polyethylene-induced osteolysis. It is more difficult to perform comparable studies to understand the biological mechanisms involved in reactions to metal particles, because, as discussed later, several aspects of metal debris present special challenges for the study of their bioreactivity.
Historical Background
The late Professor Hans Willert proposed a possible biological mechanism of aseptic loosening, namely, that continuous release of large volumes of particles quickly overwhelms the joint clearance mechanisms, and accumulated particles induce a foreign body reaction.7 According to his proposed mechanism, the particles were ingested by macrophages, which triggered the production of inflammatory cytokines, leading to bone resorption and implant loosening. At one point, bone cement was thought to be the main source of debris,8 but the introduction of cementless devices did not solve the problem of aseptic loosening or osteolysis.9
Retrieval analysis of hip resurfacing components with osteolysis of the femoral neck and the resurfaced head contributed to the understanding that polyethylene rather than cement or titanium was the culprit.10 In contrast to stemmed prostheses, which generally are removed from the bone and any osteolytic membranes at the time of revision, hip resurfacings retain the bone-membrane interfaces and facilitate their histologic evaluation. By using a special stain that showed intracellular aggregates of PE particles that otherwise were invisible at the light microscope level, we showed the extensive distribution of PE particles even when titanium metallosis was present.4,10
Cellular Mechanisms of Osteolysis
Improved understanding of the process of wear debris–induced osteolysis came when methods to isolate and characterize PE wear particles from tissues and hip simulator lubricants were developed by our group and others.3,6 We reported that the particles were predominantly micron to submicron in size, with both elongated and rounded shapes, and that these features appeared to make them particularly attractive to macrophage phagocytosis.
Molecular details of the processes involved in macrophage phagocytosis, cytokine production, and osteoclast activation have been investigated over the ensuing years, and our understanding has greatly improved. Several excellent reviews document those studies.11–13 The characteristic periprosthetic tissue response to particulate polyethylene can be summarized as follows:
2. The biological reaction is strongly determined by the concentration, size, material, and form of the wear particles; although individual variability in the extent of osteolysis has been noted, a threshold amount of wear related to risk for osteolysis has been established (0.3 mm/yr).14
It is on the basis of this knowledge that we have come to realize how much local and systemic reactions to cobalt-chromium (Co-Cr) wear products differ from all that we have learned about the periprosthetic tissue response to particulate debris of polyethylene, as the following sections will demonstrate.
Analysis of First-Generation Metal-On-Metal Total Hips
Radiographic and retrieval analyses of long-term surviving first-generation MOM bearings have shown very low annual wear of the bearings and minimal osteolysis.15 In comparison with the histiocytic inflammation typifying metal-polyethylene hip tissues, we reported that the local tissue response to well-functioning MOM bearings was markedly less inflammatory, with mostly fibrous tissue containing few macrophages.16 This was especially clear from postmortem analysis of a hip joint implanted with a McKee-Farrar hip for 30 years. A thorough histopathologic examination of tissues was performed.17 Samples from periprosthetic capsule, interface membrane, inguinal lymph nodes, kidney, spleen, and liver were examined for the amount and type of wear debris and any associated tissue pathology.
Despite 30 years of use and loosening of the cemented femoral stem, tissues around the loose implant showed only a small amount of metal staining (Fig. 12-1). Histologically, a mild histiocytic response and minimal apparent metal and minimal lymphocytes were noted in the periprosthetic tissues (Fig. 12-2). In the reticuloendothelial tissues, cobalt- and/or chromium-based particles large enough to be visible at the light microscope level were rarely seen, although particles of environmental origin were present. With the use of energy-dispersive x-ray analysis (EDAX), none of the particles in any of the organ tissues could be identified as cobalt-chromium alloy, except for one particle in a phagocytic cell within the liver. Lymphocytes and plasma cells were rare, and polymorphonuclear leukocytes were absent. No abnormalities were noted in any of the organ samples, despite the presence of high levels of cobalt (average of the four specimens was 119 ng/g; control tissue 27 ng/g) and chromium (average 105 ng/g; control undetectable) ions in the liver.
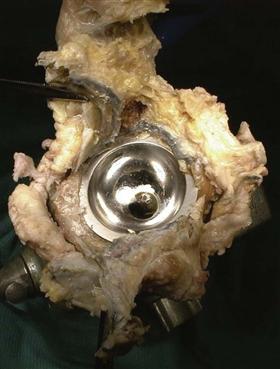
Figure 12-1 The removed acetabular component from an autopsy-retrieved McKee-Farrar, 30 years postoperatively. Note the small amount of metal staining (metallosis) within the inner capsule. Overall wear amounted to a few microns per year.
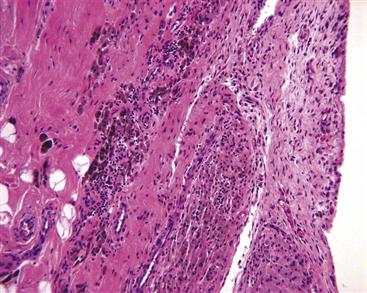
Figure 12-2 Light micrograph of periprosthetic tissue from Figure 12-1 showing mild histiocytic infiltration of the synovial lining. Phagocytic cells contain dark hematin pigment and occasional metal particles. Only a minimal lymphocyte response is seen (hematoxylin and eosin [H&E], ×200).
If it is presumed that sampled tissues were representative of the particle burden of the organ as a whole, absence of detectable particles suggested that (1) migration away from the joint was limited, (2) particles that had migrated were too small to be detected using the methods employed, or (3) they had already dissolved. It was thought initially that benign effects seen in this case were a result of the greatly reduced volume of debris alone. However, because cobalt-chromium particles are significantly smaller than PE particles, these particles most likely enter cells without inducing the inflammatory cytokine pathways associated with phagocytosis of particles of polyethylene. This is just one of the ways that biological responses to cobalt-chromium wear debris are more complicated than those involved with polyethylene.
How Cobalt-Chromium Products Are Formed
Examination of particles produced under the ideal bearing conditions found in a hip simulator reveals that cobalt-chromium particles are both globular and needle shaped.18 Electron microscopic studies have suggested that globular wear particles result from torn-off nanocrystals, and that needle-shaped particles are generated by fractured epsilon-martensite structures within the outermost layers of the metal component.19 Wear particles comprise the alloy material and oxides from the articulating surface, particularly chromium-rich oxides of the outer passivation layer and organometallic phosphates, which are deposited from the synovial fluid.20 Wimmer and colleagues described a tribolayer less than 200 nm thick that consisted of a nanocrystalline mixture of metal and organic material.21
Metal wear particles are known to be nanometer-sized, and recent improvements in isolation and examination techniques have shown that their size is affected by the specific wear mechanism responsible for their production.22 Adhesive and abrasive wear processes release billions of particles per year into the synovial tissues and fluid.23 In some conditions, this can lead to tissue staining (metallosis) (see Figs. 12-1 and 12-3). Under more severe wear conditions, such as edge loading, the stable protective chromium oxide layers found on the outermost surfaces can be damaged or removed, producing larger particles and leading to local crevice corrosion, pitting, and dissolution of metal in areas of damage.24,25
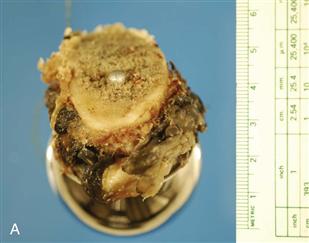
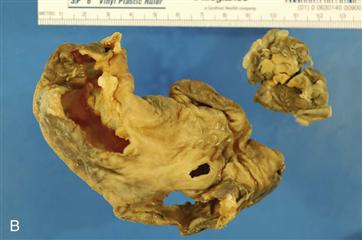
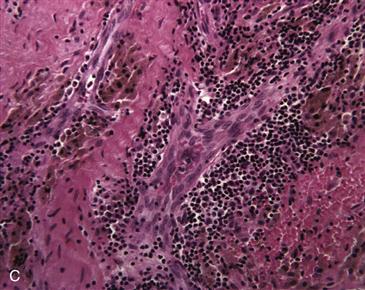
Figure 12-3 Examples of metallosis. A, Hip resurfacing arthroplasty revised for acetabular malposition after 22 months, seen from below to highlight the metal-stained villous tissue adhering to the femoral neck bone. B, This enlarged tissue bursa was removed from a metal-on-metal hip resurfacing revised for acetabular malpositioning, femoral osteolysis, and pain after 56 months. This bursa contained several hundred milliliters of brown fluid under pressure. C, Histology of this bursa tissue shows a mix of dark macrophages containing hematin and metal, and lymphocyte aggregates containing plasma cells (hematoxylin and eosin [H&E], ×200).
Although most of the particles entering the joint are produced from the bearing, it must be remembered that nonarticulating surfaces can contribute to the particle burden. For example, the taper connections of modular total joint replacements can be an important source of metal wear and corrosion products (Fig. 12-4).26 Collier and coworkers27 examined the tapered interface between the head and neck of total hip replacements and reported that in 91 cases in which the same alloy was used for the femoral head and stem, no corrosion products were found. In contrast, corrosion products were detected in 25 of 48 prostheses with a titanium alloy stem coupled with a cobalt-chromium head. The potentially inflammatory nature of these corrosion products has also been noted.28,29
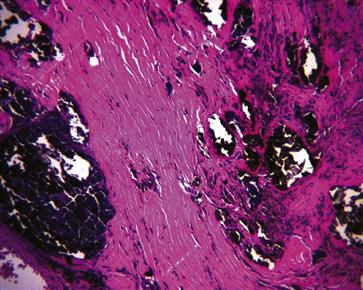
Figure 12-4 Histologic micrograph of solid corrosion products in periprosthetic tissue taken from a modular total hip replacement with signs of corrosion on the stem taper. Only a mild histiocytic response to this material is noted (hematoxylin and eosin [H&E], ×200).
The Fate of Particles Entering the Joint
Wear particles are released into the joint fluid, where they may come into contact with phagocytic cells, primarily of the macrophage lineage, and become internalized by phagocytosis or pinocytosis, depending on their size. Although some particle-laden cells may be distributed away from the joint via the vascular and lymphatic systems,30,31 many remain in the local tissues.32 This results in the accumulation of particles within synovial phagocytic cells and histiocytes in the tissues lining the joint (Fig. 12-5). Particles can also be stored freely in the interstitial fluids, in synovial fluid, and in fluids that can accumulate within tissue bursae. Additionally, particles can be bound up in fibrin-lining bursae, in joint tissues, or within bone cysts.
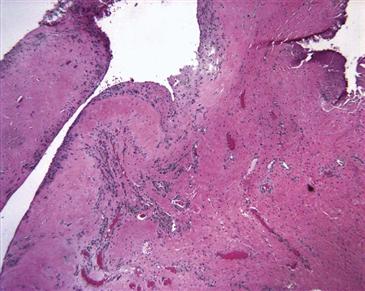
Figure 12-5 Histologic micrograph of synovial tissue from a case revised with metallosis shows discolored macrophages containing metal particles at the tissue edge. In this example, no lymphocytic response is obvious (hematoxylin and eosin [H&E], ×100).
When ingested by phagocytes, Co-Cr particles are exposed to the aggressive intracellular environment of lysosomes and phagosomes, where pH values can be as low as 4.6.33 This will accelerate corrosion of metallic debris, locally increasing cobalt and chromium ions to potentially toxic levels. When the cell dies, particles are spilled back into joint tissues, where they may enter joint fluid or be rephagocytosed to continue the cycle locally, if the cell remains in the joint, or at a distant site, if the cell migrates through the vascular or lymphatic system.
Metal particle migration from total hip replacements with metal-polyethylene bearings was studied in a group of 30 autopsy retrievals collected after an average of 5.8 years (range, 3.6 to 14.3 years) from patients ranging in age from 43 to 91 years.34 Light and electron microscopy and identification of particle species through x-ray microanalysis demonstrated submicrometer metal particles within macrophages in the liver and/or the spleen in 11 of 15 patients with a revised arthroplasty and in 2 of 15 patients with a primary hip replacement.
Among patients with a revised hip replacement, particles were present in the spleen alone in five patients, in both the liver and spleen in another four patients, and in the liver alone in two. Particles had been generated from nonbearing surfaces such as loosened components, ancillary fixation wires, plates or screws, and a well-fixed acetabular component and its fixation screws. In patients with primary hip replacement, metal particles generated between nonbearing surfaces were detected in the liver of one patient and in the spleen of another patient. The size of disseminated particles ranged from 0.1 to 8 µm, with most particles measuring less than 1 µm.
Particles were phagocytosed by macrophages, which formed focal aggregates in the organs without apparent toxicity. In the spleen, macrophages containing metal particles were found primarily within the lymphatic sheaths surrounding arterial vessels, where they formed foreign body granulomas. In the liver, metal particles were found in focal clusters in macrophages of the portal tracts and were distributed around venules in the parenchyma. These particles were commonly mixed with particles from environmental origins such as silicates and particles containing titanium or aluminum.
From these observations, it is clear that both local and systemic effects of cobalt-chromium particles must be considered. The following discussion will review what is known about cobalt and chromium wear and corrosion products in the context of patients with MOM hip replacements. In particular, important factors influencing the measurement of metal ions and the effects of large quantities of wear products will be discussed.
Cobalt and Chromium Ions in Patients with Metal-On-Metal Implants
The billions of nanometer-sized metal particles have an extremely high surface area, facilitating corrosion processes producing products that become detectable in blood, urine, and other bodily fluids as cobalt and chromium ions. Depending on the chemistry of the periparticulate fluid, various corrosion products can be produced, including soluble and insoluble forms of various salts and metal-protein complexes, free radicals, and reactive oxygen species.35,36 These will vary in size, stability, and solubility, and consequently in their bioavailability.37–39 However, when a sample of blood and urine is tested using atomic absorption or inductively coupled plasma mass spectrometry, these circulating corrosion products are detected as cobalt and chromium ions.
Systemic responses to circulating ions and nanometer-sized metallic particles debris are still not well understood, but concerns have been raised that the long-term consequences may be dire.40 Since the time that cobalt-chromium alloy bearings were first implanted, long-term local and systemic effects of the particulate, and of ionic forms of the resulting wear debris or corrosion products, have been matters of concern.41,42 Reports of sustained elevations in circulating metal levels and of the presence of chromosomal aberrations in joint tissues from MOM patients added to this concern.43,44 A small-scale epidemiologic study that looked for associations between cancer rates and MOM bearings reported slight elevations in certain types of cancers.45 Later follow-up involving comparison of the causes of death among 579 patients with McKee-Farrar MOM components and Brunswick or Lubinus metal-polyethylene prostheses noted that cancer mortality in the McKee group was similar to that of the general population,46 although it was recognized that any detectable increase in cancer would require a large-scale survey for several decades to account for the anticipated 20- to 30-year latency period.47
Metal Wear Products and Cancer
Concern that cobalt-chromium wear products could cause cancer in patients is based on the known carcinogenic potential of these metals, particularly chromium in the hexavalent form (CrVI), and observations that exposure to cobalt and chromium ions can cause aberrations in cellular DNA.44,48,49 It is extremely difficult to determine whether particles produced in an MOM bearing exist in the trivalent or hexavalent form. Chromium in its hexavalent form can enter cells much more readily than in its trivalent form. It is thought to bind mostly to nucleoproteins.50 The intracellular process of reduction of hexavalent chromium to the more stable trivalent form is thought to be harmful to chromosomes and other intracellular organelles.43
Chromosomal changes have been demonstrated in patients with various types of joint replacements, including metal-on-polyethylene and MOM devices.48,51 A statistically significant increase in both chromosome translocations (relocation of a chromosomal segment in a different position in the genome) and aneuploidy (chromosome loss and gain) was noted in the peripheral blood lymphocytes of 95 patients with Metasul MOM (Zimmer, Warsaw, Ind) total hips at 6, 12, and 24 months after surgery.49 No statistically significant correlations between chromosome translocation indices and cobalt or chromium concentrations were reported, but they did correlate with molybdenum levels despite their low levels. Similar aberrations were shown to occur with titanium; the clinical implications of this and other studies are as yet unclear, as no case of an implant-induced cancer has been confirmed to date.52–54 The multifactorial nature of DNA damage makes linking the results of these studies to a clinical problem very difficult.55
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








