Chapter 28 Arthroscopic Treatment of Degenerative Arthritis of the Knee
Historical Perspective
Arthroscopic débridement for OA of the knee was initially reported by Burman and colleagues20 in 1934. They reviewed the first 30 cases in which knee arthroscopy was used to diagnose a “possible meniscal injury, arthritis in the knee, or suspected tumor.”19,20,28 In the group of arthritic cases, they had “the pleasant surprise of seeing a marked improvement in the joint following arthroscopy.” They noted that “arthroscopy involves only minimal risk, and in some cases has actually had a beneficial therapeutic effect, probably due to the thorough flushing and distention of the joint which it necessitated.” In 1941, Magnuson introduced the term joint débridement to describe an operation of the knee in which “all the accessible synovial membrane, osteophytes, diseased cartilage, and normal soft tissues were removed in an effort to relieve the symptoms of osteoarthritis”; this was performed as an open procedure in which “complete recovery of symptoms” occurred in 60 of 62 patients.51
During and after World War II, arthroscopy waned, and the open Magnuson procedure consisting of total synovectomy, osteophyte resection, cruciate ligament excision (if torn), as well as patellectomy was performed in most cases, with reported symptomatic improvement in 66% of patients. This procedure became widely accepted as the treatment of choice for OA of the knee as reported by Haggart33 in 1947 and Isserlin41 in 1950. These open débridement procedures, therefore, became the treatment of choice for arthritis of the knee until the resurgence of arthroscopy in the early 1970s.
Cartilage Repair
In 1743, William Hunter stated that “from Hippocrates to the present age, it is universally allowed that ulcerated cartilage is a troublesome thing and that once destroyed it is not repaired.”39 In 1849, Leidy confirmed this principle, stating that “a rupture of cartilage fragments is never united and that articular cartilage lacks regenerative power and fracture gaps extending into the joint become filled with tough fibrous tissue”42,48
Redfern, in 1851, described the histology of induced wounds of the articular cartilage of dog joints and stated that the wound “healed perfectly by the ingrowth of fibrous tissue,”71 which he believed arose from the intercellular substance of the chondrocytes of the articular cartilage. However, as Mankin concluded in 1952, superficial lacerations of cartilage “neither heal nor progress to more serious disorders if they are small lesions.”52 On the basis of multiple animal studies, these superficial lacerations, therefore, are generally limited in progression and do not lead to clinical osteoarthritis. It was further noted that deep lacerations may be clearly visible years after injury.53–55 When the subchondral bone is thus disrupted, intraosseous blood vessels expose bone matrix growth factors, causing fibrin clot formation. Inflammation introduces new cells into the cartilage defect and these cells proliferate and begin matrix repair.16
The native matrix of articular cartilage has extraordinary biochemical characteristics. It is a hyperhydrated tissue, with estimates of water content ranging as high as 80%. Connective tissue contains type I collagen, consisting of two alpha and one alpha-2 chains. The type II collagen of articular cartilage contains three alpha-1 chains. Furthermore, the alpha-1 chains of type II collagen have a different structure from those of type I. It is this type I collagen that is formed when fibrous tissue regenerates in attempts to repair injured articular cartilage.* Furthermore, mature fibrocartilage repair tissue has a relatively low proteoglycan concentration, and the proteoglycans do not resemble the large elaborate molecules found in native articular cartilage. Therefore, the healing response does not produce tissue with the unique composition, structure, and biochemical properties of normal articular cartilage.16,62
After cartilage injury or during the progression of osteoarthritis, some chondrocytes do proliferate but do not migrate through the matrix to enter the site of tissue injury. Any repair tissue matrix is formed by undifferentiated cells arising from the bone marrow and contains primarily type I collagen; thus, the normal articular cartilage properties cannot be restored. These reparative cells fail to organize the molecules they produce to create a strong cohesive structure similar to that of articular cartilage, and they produce other types of molecules that may interfere with the assembly of the cartilage matrix. This abnormal matrix, with its different composition and structure, therefore, adversely alters the material properties of the tissue.26,69,72 These alterations compromise the ability of cartilage to survive and function in the highly stressed mechanical environment found in load-bearing joints and may lead to further cartilage degeneration and osteoarthritis. Disruption of collagen cross-linking causes cartilage to lose its intrinsic tensile stiffness, strength, and shear stiffness; the loss of proteoglycans and increased water content compromise its compressive and permeability properties.4,56,80
Marrow Stimulation Procedures
The concept of drilling through eburnated bone to stimulate reparative cartilage formation was originally described by Pridie in 1959 (Fig. 28-1); of 62 patients, 74% (46) believed that their operation was a success and stated that they would “have the operation again under similar circumstances.”68 To reconfirm these findings, Akeson surgically removed the articular cartilage of the femoral heads of dogs and drilled the subchondral bone. It was noted that after 1 year, at the time of retrieval, “excessive loading destroyed the initial repair tissue or prevented formation of repair tissue.”2 The results also indicated that 1 year after surgery, the concentration of proteoglycans in the reparative cartilage was less than half of that found in normal cartilage. Mitchell and Shepard58,59 found that multiple small drill holes made in the subchondral bone of rabbit knee joints stimulates repair from large areas of the articular surface. They determined that repair tissue grows from the drill holes and spreads over the exposed bone. However, large areas of repair tissue that initially had the appearance of hyaline cartilage begin to fibrillate and deteriorate within 1 year. These experiments were the first to show that abrasion or perforation of subchondral bone could stimulate repair of large areas of joint surface with fibrocartilaginous tissue, but the retrieved repair tissue lacked the proteoglycan concentration found in previous studies of normal hyaline cartilage.
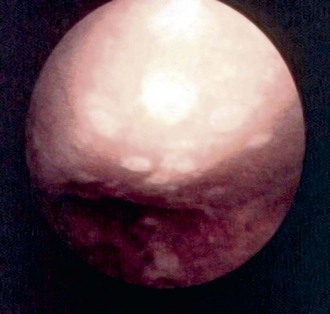
Figure 28-1 Pridie procedure illustrating fibrocartilage formation in medial femoral condylar drill holes.
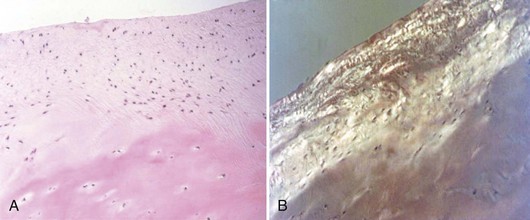
Abrasion arthroplasty of grade IV eburnated chondral lesions using motorized instrumentation was introduced by Johnson in 1981. This procedure is essentially an extension of the Pridie procedure except that in abrasion arthroplasty, a superficial layer of subchondral bone, approximately 1 to 3 mm thick, is removed to expose interosseous vessels (Fig. 28-2). Theoretically, the resulting hemorrhagic exudate forms a fibrin clot and allows for the formation of fibrous repair tissue over the eburnated bone (Fig. 28-3). In some patients, this fibrocartilaginous tissue lasted up to 4 years but in Johnson’s series, only one of eight biopsy specimens showed any type II collagen typical of hyaline cartilage at the time of arthroscopic review and biopsy, and the rest had types I and III collagen.9,45 In a series of patients at our institution who had abrasion arthroplasty, at 5-year follow-up examinations, 15 had been converted to total knee replacement (TKA) and biopsies were obtained at the time of TKA. All patients had fibrocartilage and type I collagen in their biopsy specimens (Fig. 28-4).
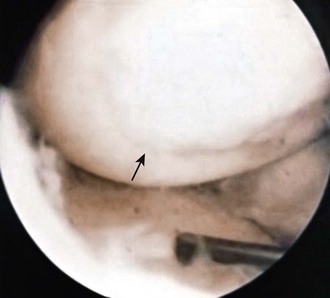
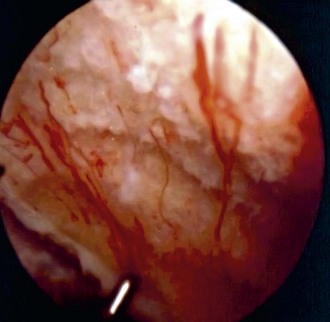
Figure 28-3 Arthroscopic view of patient 4 years after abrasion arthroplasty showing resurfacing with fibrocartilage.
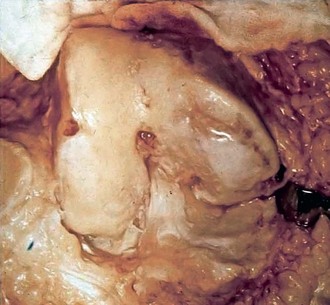
In our series of 126 patients who had treatment of unicompartmental gonarthrosis with abrasion arthroplasty or arthroscopic débridement alone, at 5-year follow-up examinations, 51% had good to excellent results with abrasion arthroplasty; 66% had good to excellent results with arthroscopic débridement alone.9 However, all these patients had complete obliteration of the medial joint space preoperatively. The results in our series were unrelated to age, presence of previous surgery, weight, extent of unicompartmental disease, presence or absence of joint space widening after surgery, and extent of residual varus or valgus deformity. Coventry and Bowman23 noted that formation of hyaline-like cartilage occurred in the unloaded medial compartment of several patients after valgus upper tibial osteotomy (Fig. 28-5). This finding was confirmed arthroscopically by Fujisawa and associates30 12 to 18 months after upper tibial osteotomies, which implies that regeneration of reparative cartilage can occur secondary to unloading of bone alone, without additional surgery.
Microfracture
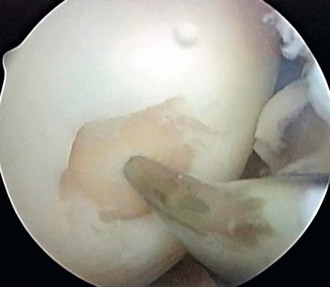
Blevens and coworkers12 have recommended a microfracture technique in which they use an arthroscopic awl to create multiple perforations into the subchondral bone arthroscopically. They reported on 266 patients between 1985 and 1990, with a 3.7-year follow-up using a similar grading system to the Outerbridge classification.66,73 The indications for the microfracture technique include a full-thickness, well-circumscribed cartilage defect on a weight-bearing surface of the knee, with exposed subchondral bone (i.e., grade IV lesions). After chondral surface débridement, the bone is perforated to a depth of 3 to 4 mm using an awl, with the holes placed approximately 4 to 5 mm apart (Fig. 28-6). Blood should be seen emanating from the microfracture holes after perforation is complete. A postoperative rehabilitation program was used to provide motion without applying high load stress to the treated chondral defect. Repeat arthroscopies were performed in 80 patients. In most chondral defects, subchondral bone was covered with cartilage of varying quality and the term hyaline-like was introduced to describe the fibrocartilage surface. There was no evidence that hyaline cartilage was present at the second-look arthroscopy, and the authors confirmed that the only type of tissue that was seen to regenerate over these surfaces was fibrocartilaginous repair tissue. Furthermore, they stated that they were unable to determine the biochemical composition and durability of the presumed fibrocartilage repair tissue. Clearly, there is no evidence that hyaline cartilage is regenerated by marrow stimulation.
Arthroscopic Débridement
Arthroscopic débridement as a treatment option for OA was initially reported by Sprague in 1981, who arthroscopically débrided 330 knees diagnosed as having “degenerative arthritis … in two or more compartments of the knee.”76 Meniscectomy, chondroplasty of all surfaces, and loose body and debris removal were performed. At 1-year follow-up, 74% of these patients stated that the “knee was improved and more functional” than before surgery. The extent of arthritis, however, was not correlated clinically or roentgenographically with success rates. In the early to mid-1980s, others also reported that the results of arthroscopic débridement were not correlated with age or the extent of arthritis, either roentgenographically or arthroscopically, with up to 11-year follow-up.44,75 However, Gross32 and Ogilvie-Harris and Fitsialos65 concluded in 1991 that OA severity was the best predictor of success after arthroscopic débridement and that normally aligned knees with mild arthritis had the best results with 8-year follow-up.
It is certainly not clear, however, that shaving damaged articular cartilage relieves pain. O’Donoghue64 reported that chondroplasty in rabbit knees did not stimulate cartilage repair nor did it result in joint deterioration. Bentley7,8 reported that chondroplasty during arthrotomy produced unpredictable results, and only 25% of patients treated with patellar chondroplasty had satisfactory results beyond 1 year. Timoney and colleagues78 retrospectively reviewed 109 patients who had arthroscopic débridement for degenerative arthritis of the knee, with 4.2-year follow-up. Only 45% reported good results; 21% of patients experienced worsened symptoms and subsequently underwent TKA.
In 1996, Moseley60 was one of the first to suggest that arthroscopic débridement for OA of the knee was no better than placebo. In that study, 10 patients with OA of the knee were randomized into a placebo group, an arthroscopic lavage group, and an arthroscopic débridement group. All patients at 6 months reported improvement in their pain scores and satisfaction with their surgery with the exception of one placebo patient. This study was repeated in 200261 with a larger patient group at a Veterans Administration hospital; 70% of these patients had moderate to severe OA. No significant differences were found among the three groups who had arthroscopy with débridement, arthroscopy with lavage, and placebo knee surgery. Those patients who had positive magnetic resonance imaging (MRI) scans with meniscal tears were excluded from the study. It was concluded that there is no clear role for arthroscopy in knees with OA.
Steadman and associates77 recently reported a 71% success rate at 2 years for arthroscopic débridement of OA using the Western Ontario and McMaster Index (WOMAC) and Lysholm scoring systems. Wai and coworkers79 and Hawker and colleagues37 reported that up to 9.2% of patients had a TKA after 1 year and 18.4% had a TKA after 3 years subsequent to arthroscopic débridement, indicating the transient nature of improvement in some patients undergoing this procedure. A number of studies have claimed that arthroscopic débridement and shaving help relieve the symptoms of OA of the knee, but it is unclear why these patients improve and why they remain improved for as long as 5 years postoperatively.*
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree