CHAPTER 102 Arachnoiditis and Epidural Fibrosis
Spinal arachnoiditis is a nonspecific inflammatory process of the arachnoid layer of the spinal cord or cauda equina. Arachnoiditis was first described by Victor Horsley in 1909.1 Since Horsley, numerous authors have described it with a variety of terms including: chronic spinal arachnoiditis, adhesive spinal arachnoiditis, meningitis serosa circumscripta spinalis, chronic spinal meningitis, spinal meningitides with radiculomyelopathy, lumbar adhesive arachnoiditis, spinal arachnoiditis, spinal fibrosis, and lumbosacral adhesive arachnoiditis. Furthermore, on the basis of specific radiographic or pathologic findings, arachnoiditis can be termed arachnoiditis ossificans, calcific arachnoiditis, or pachymeningitis.1
Anatomy
The arachnoid mater is an avascular membrane that lies between two vascularized membranes, the pia mater and dura mater. The arachnoid is attached to the underlying pia by numerous arachnoid trabeculae, creating a space between the arachnoid and the pia.2 This space, or potential space in some instances, transmits arterioles and is referred to as the subarachnoid space. The arachnoid is composed of layers of squamous cells held together by a network of connective tissue. The arachnoid contains intercellular pores, which allow for the passage of molecules.3
Pathogenesis
A chronic infection or irritation can cause the arachnoid membrane to become thickened and adherent to both the overlying dura mater and the subjacent pia mater.4 The pia-arachnoid carries the blood vessels to the spinal cord and this layer contains mesenchymal cells. In 1951 Smolik and Nash recognized that when the outer arachnoid layer is injured, both the blood vessels and mesenchymal cells lend themselves to extensive proliferation. The ensuing reaction between the pia-arachnoid and the dura mater leads to obliterative arachnoiditis.5
When the arachnoid membrane is exposed to an insult, an inflammatory response ensues, characterized by fibrinous exudates, neovascularization, and a relative paucity of inflammatory cellular exudates.6,7 Vascular occlusive changes can lead to spinal cord ischemia.4,8–11 The small perforating blood vessels that supply the portions of the white matter may be obliterated with resultant necrosis and cavitation of the spinal cord parenchyma.8,9,11 In addition to ischemia, blockage of venous return from the spinal cord or occlusion of cerebrospinal fluid (CSF) pathways may occur.8
The stages of progressive inflammation of the arachnoid that occur in lumbosacral arachnoiditis were described by Burton. The initial stage, radiculitis, consists of an inflamed pia-arachnoid with associated hyperemia and swelling of the nerve roots. The second stage, arachnoiditis, is characterized by fibroblast proliferation and collagen deposition. During this stage, nerve root swelling decreases and the nerve roots adhere to each other and to the pia-arachnoid. The final stage, adhesive arachnoiditis, is the resolution of the inflammatory process and is characterized by dense collagen deposition. There is marked proliferation of the pia-arachnoid, as well as complete nerve root encapsulation, hypoxemia, and progressive atrophy.12 For reasons that are not fully understood, the adhesions occur preferentially on the dorsal segments.1 The exact time course of these three phases has not been elucidated. Furthermore, it is not known how the specific causative insult for the development of arachnoiditis might affect the time course of each of the three phases.
Yamagami and colleagues13 postulated that the pathologic changes in arachnoiditis may be secondary to diminished nutritional supply. They found that, in an experimental rat model, the development of arachnoiditis and neural degeneration directly corresponded to the magnitude of extradural inflammation and wound healing processes that occurred after laminectomy, with or without foreign bodies. Furthermore, adhesions of the arachnoid cause the nerve roots to lump together and, in doing so, these nerve roots are isolated from contact with the CSF, with resultant nutritional compromise.13
Etiology
In the first half of the 20th century, arachnoiditis was most often attributed to infectious causes.8 Furthermore, arachnoiditis had been described mainly in the cervical and thoracic regions.1 Since the 1950s, there has been a trend toward a higher incidence of arachnoiditis of noninfectious origin affecting the lumbar region.1,8 The precise causes of spinal arachnoiditis are not clear, and likewise, the incidence and prevalence of spinal arachnoiditis in the general population is unknown.8
As stated previously, arachnoiditis was mainly of infectious origin in the first half of the 20th century. Syphilis, tuberculosis, and gonorrhea were the most prevalent causes.1,14 Less common infectious causes include parasitic diseases and viral meningitis.15,16 These infectious causes are important to differentiate from noninfectious causes of arachnoiditis because, in most cases, effective treatment is available for arachnoiditis of infectious origin. However, despite adequate treatment of the causative agent, scarring of the arachnoid membrane may lead to permanent damage.
There are a number of important noninfectious etiologies of arachnoiditis. In the 1940s, blood in the CSF following subarachnoid hemorrhage or surgery became the most prevalent cause of arachnoiditis.1 Spinal arachnoiditis following subarachnoid hemorrhage continues to be common and is usually treated in a conservative fashion.17 The breakdown products of hemoglobin form free radicals, and it has been postulated that these cause damage to nerves.18,19 In experiments on dogs, it has been shown that injecting blood breakdown products into the subarachnoid space causes more meningeal inflammation than does the injection of fresh blood.18 Cases of patients who have received epidural blood patches have given controversial results. Digiovanni and colleagues20 described that the placement of an autologous blood patch into the epidural space produced no more inflammation than a standard lumbar puncture. Other authors, though, have described cases where an epidural blood patch had allegedly been responsible for arachnoiditis.21 Abouleish and colleagues22 described 118 cases of epidural blood patches over a 2-year period. This group found 19 cases of axial back pain, two cases of radiculopathy, and no cases of arachnoiditis.22
Oil-based contrast media have been historically important causes of arachnoiditis. Iophendylate (Myodil, Pantopaque) is an oil-based contrast medium used in diagnostic myelograms. It was first used in the United States, in 1944, and its usage continued for 40 years. In Sweden iophendylate was banned from clinical use, in 1948, secondary to animal studies that identified it as a causative agent for arachnoiditis.23 The incidence of arachnoiditis after the use of iophendylate is dose dependent and is quoted as 1%.24 Iophendylate has a long half-life, so it is usually removed from the thecal space, by aspiration, at the conclusion of the myelogram.8 Often, this removal process is not entirely successful, and in fact, incomplete removal of the contrast dye may produce further trauma and cause bleeding into the CSF.4
Guyer and colleagues25 listed the following factors as influencing the development of arachnoiditis after myelography: the type of contrast agent used (the risk is greater with the oil-based medium than with the water-soluble medium and greater with the ionic medium than with the nonionic medium), the dosage of contrast medium, and the observation time after myelography (Fig. 102–1).
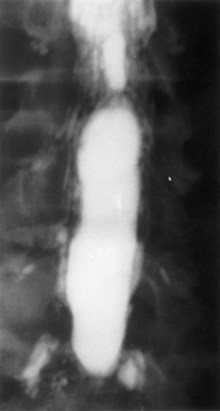
FIGURE 102–1 Myelogram with oil-based medium demonstrating the marked lack of filling of nerve roots of adhesive arachnoiditis.
The use of intrathecal medications, either steroids or anesthetic agents, has been implicated as a cause of arachnoiditis. Intrathecal injection of corticosteroids was previously used for multiple sclerosis.8 Epidural injection of corticosteroids for back pain is a common practice. One of the most commonly used agents is methylprednisolone acetate (MPA), which has been reported to cause arachnoiditis.26–28 MPA is suspended in polyethylene glycol, which can cause arachnoiditis.26–28 Furthermore, MPA is known to easily cross the intrathecal space, thus causing arachnoiditis.28 Animal studies, though, have not shown MPA to cause significant meningeal inflammation after epidural injections.29–31
The use of intrathecal bupivacaine, with or without epinephrine, has also been reported to cause arachnoiditis. Boiardi and colleagues32 described several cases of arachnoiditis after administration of bupivacaine with epinephrine. Gemma and colleagues33 described a case of arachnoiditis after intrathecal administration of bupivacaine without epinephrine. It is unclear in these cases whether the arachnoiditis was triggered by the bupivacaine or other preservatives. Furthermore, it is unclear whether epinephrine plays a role in the pathogenesis of arachnoiditis.
A history of spinal surgery is a risk factor for arachnoiditis.8 In particular, some investigators have specifically stated that surgery for a herniated intervertebral disc may lead to arachnoiditis.5,7,25 Carroll and Wiesel34 showed that a postoperative pain-free interval lasting between 1 and 6 months, followed by the gradual onset of leg pain, increases the likelihood that some scar tissue is responsible for the symptoms. Smolik and Nash5 showed that simple dural retraction for the visualization of a ruptured intervertebral disc may trigger arachnoiditis. Haughton and colleagues35 showed that, in monkeys, the nucleus pulposus of an intervertebral disc was able to cause focal arachnoiditis.
Clinical Features
The diagnosis of arachnoiditis requires a detailed medical history and physical examination, as well as a review of confirmatory radiographic imaging studies. In obtaining a medical history from a patient with arachnoiditis, the clinician should seek three major characteristics of the pain. Pain of arachnoiditis is typically described as a burning pain that is constant and worsened by activity.12 The pain of arachnoiditis may be located in the back or the lower limbs or both. The symptoms of arachnoiditis can vary from nonspecific back pain to radiculopathy and myelopathy.36 Intractable pain that occurs secondary to arachnoiditis has a poorly localized pain pattern that is diffuse. In many patients, arachnoiditis is asymptomatic and is discovered as an incidental radiographic finding.37 The pain symptoms of chronic arachnoiditis may be similar to those of other chronic pain syndromes such as Complex Regional Pain Syndrome. The exact relationship of these pain syndromes has not been fully elucidated.
The physical examination findings in patients with arachnoiditis have been reviewed in two large clinical series. Burton followed 100 patients with arachnoiditis and found little motor weakness to be present. These patients were commonly found to have a positive straight leg raise sign, a tender sciatic notch, limited range of motion of the trunk, and paravertebral muscle spasms.12 Guyer and colleagues25 followed 51 patients over more than 10 years and found that a decreased range of motion of the trunk was the most common finding on physical examination. In cases of chronic arachnoiditis with resultant syrinx formation, physical examination findings of syringomyelia are present. These include dissociative sensory loss and variable long tract signs.8









