Chapter 49 Antimalarial Medications
Introduction
Paine first used quinine for the treatment of discoid lupus erythematosus.1 It was not, however, until 1951, when Page used quinacrine (Mepacrine) in discoid lupus erythematosus (DLE), rheumatoid arthritis, and systemic lupus erythematosus (SLE), that antimalarial (AM) medications became widely used. The AM compounds hydroxychloroquine (HCQ) and chloroquine (CQ) and, to a lesser extent, quinacrine (also called Atabrine in the United States) remain in use.
Pharmacokinetics of Antimalarial Medications
Hydroxychloroquine
HCQ is a 4-aminoquinoline. Between 75% and 100% is absorbed, and 50% is eventually bound to serum proteins. Excretion occurs in a rapid phase with a half-life of 3 days and a slower phase with a half-life of 40 to 50 days.2 Approximately 45% is excreted by the kidney, 3% by the skin, and 20% by the bowel. Renal excretion of HCQ can be enhanced by acidification of the urine. Steady-state plasma levels are reached after 6 months of therapy.
Mechanisms of Action
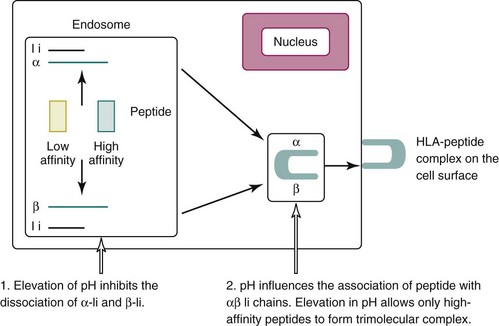
In 1993, Fox2 proposed that AM medications modulate the immune system through their known ability to influence pH in intracytoplasmic vesicles. By increasing the pH, AM drugs inhibit the processing and assembly of self-peptides into complexes with major histocompatibility complex (MHC) class II proteins. This results in decreased stimulation of CD+ T cells that are reactive to autoantigens, decreased release of proinflammatory cytokines and, ultimately, a diminution of autoimmunity (Box 49-1) (Figure 49-1).
Box 49-1
Mechanisms of Action of Antimalarial Medications
AM medications are weak bases that can pass freely across cell membranes. Once inside the acidic vacuole, the AM drug becomes protonated, and the now charged drug (hydroxychloroquine-H) is unable to pass out of the vesicle. The continuous accumulation of hydrogen ions by HCQ leads to a subtle elevation of pH within the acidic vacuole, which decreases the affinity of α-Ii and β-Ii, allowing peptides to displace the Ii and form the αβ-peptide complex (Figure 49-1). When the pH is even slightly elevated, the lower affinity peptide might not be able to displace the Ii. Cryptic autoantigens are characterized by their low affinity for self-MHC. Elevation of the pH in the loading compartment of the endoplasmic reticulum might selectively decrease the loading of autoantigen self-peptides, while leaving the response to exogenous peptides intact. Thus no increase in infections would occur with AM medications.
Increased levels of proinflammatory cytokines are believed to play a role in the pathogenesis of SLE, and AM therapy may influence their release from inflammatory cells.4,5 For example, Wozniacka and others4 demonstrated that a significant reduction in the elevated levels of serum interleukin (IL)-1β, IL-6, IL-18, and tumor necrosis factor–alpha (TNF-α) occurred after 3 months of treatment with CQ. IL-18, known as interferon gamma (IFN-γ)-inducing factor, is produced mainly by macrophages during innate immune responses and thus influences adaptive immunity. Contribution of IFN-γ to the pathogenesis of SLE has been demonstrated in animal models and is considered a major effector molecule in SLE.6 In a separate study, Wozniacka and associates5 also demonstrated the local inhibitory effect of CQ in the expression of proinflammatory cytokines in the irradiated skin of patients with SLE,5 and the reduction in human leukocyte antigen (HLA)–DR+ and CD1a+ cell numbers in both unirradiated and ultraviolet-irradiated skin.7 The latter suggests that CQ reduces the number of APCs in the skin of patients with SLE, thereby explaining the benefits for skin lupus.
Over the last several years, a paradigm shift in understanding the importance of the innate immune system in SLE has been driven by the recognition of a new class of pattern recognition receptors, collectively known as Toll-like receptors (TLRs).8 Rönnblom and Alm9 were the first to demonstrate that immune complexes containing DNA or RNA (present in SLE sera) could trigger the production of high levels of IFN-α by plasmacytoid dendritic cells via the Fc-gamma receptor, which is one of the most important receptors for inducing phagocytosis of antibody-coated microbes. Since other kinds of immune complexes did not elicit this kind of IFN-α response, the findings indicated a unique function of nucleic acids in the activation of the innate immune system. Recent evidence suggests that the ability of immune complexes to activate plasmacytoid dendritic cells depends on Fc-gamma receptor–mediated delivery to a cellular compartment containing TLR9 or TLR7.10 The concentration of CQ that is necessary to block TLR9 is in the same range as the concentration of CQ (greater than 1 µg/mL) associated with decreased frequency of SLE flare in vivo.11,12 Furthermore, in a retrospective study of 130 U.S. military personnel who eventually developed SLE and whose clinical variables in the period before diagnosis were available, James and colleagues13 found that early HCQ use is associated with the delayed onset of SLE.
TLRs recognize specific patterns of microbial components and regulate the activation of both innate and adaptive immunity. TLR3, TLR7, TLR8, and TLR9 specifically recognize nucleic acid motifs and are also distinct from other TLRs in that they are expressed not on the plasma membrane but intracellularly. This could be of particular relevance in SLE, considering the pathogenic role of anti–double stranded DNA (anti-dsDNA) antibodies. Moreover, signal transduction through these receptors depends on the internalization of the ligand to an acidic intracellular compartment. As a result, agents that interfere with endosome or lysosome acidification, such as AM medications, can block the activation process.14
Efficacy of Antimalarial Medications
Although AM drugs have been used empirically in SLE since the 1950s, evidence from controlled studies supporting their use is more recent. A systematic review on the clinical efficacy and safety of AM therapy15 noted that AM medications decreased disease activity in all studies, often by 50%.
Controlled Studies Assessing Efficacy
In 1975, Rudnicki, Gresham, and Rothfield16 reported the first controlled study of AM medications in SLE. The authors retrospectively studied 43 patients who developed a macular lesion resulting in the discontinuation of AM agents. Every year on AM drugs was matched in consecutive order to a year off AM medications. In total, 76 years could be matched (76 years on AM drugs and 76 years off). Of the 43 patients, 24 (56%) who had received high-dose CQ (500 mg/day) had a significantly lower frequency of constitutional symptoms (e.g., fatigue, weight loss, fever) and skin manifestations during the years they received CQ than the years they did not (Table 49-1). The generalizability of these results is limited because the doses used today are lower. However, because SLE disease activity tends to decrease over time and the years on AM medications always preceded the years off, this pattern biased against demonstrating the efficacy that was identified.
TABLE 49-1 Observational and Controlled Trials Assessing the Efficacy of Antimalarial Medications on Disease Activity in Patients with Systemic Lupus Erythematosus
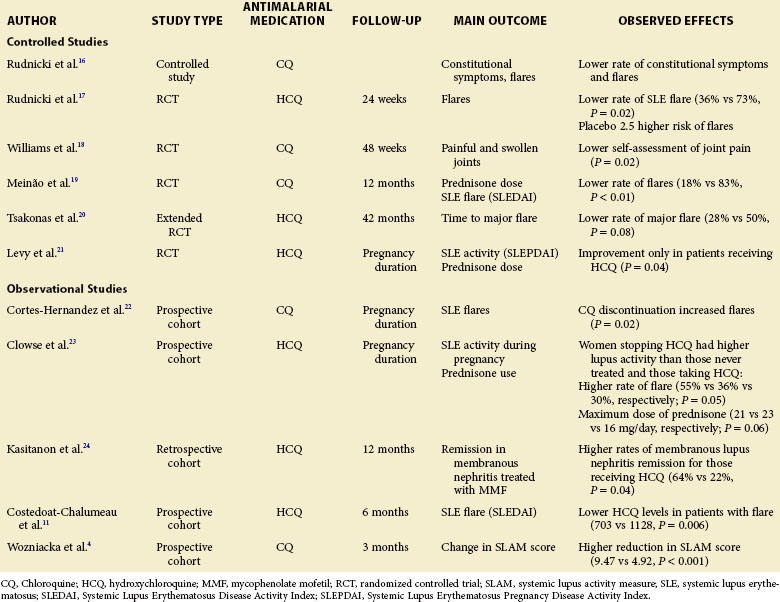
A second landmark study was published in 1991 by the Canadian Hydroxychloroquine Study Group, which reported a multicenter, placebo-controlled, double-blind randomized study of the effect of withdrawing HCQ in patients with stable SLE.17 Forty-seven patients with quiescent SLE were randomized to receive placebo or to continue with HCQ; they were followed for 24 weeks. The HCQ group had significantly fewer disease flares than the placebo group (36% versus 73%, respectively; P = 0.02). The time to flare-up was also shorter in the placebo group. Overall, patients randomized to placebo were 2.5 times (95% confidence interval [CI] 1.08-5.58) more likely to have mild clinical flares than those who remained on HCQ. Five of the patients taking placebo (23%) and 1 who continued to take HCQ (4%) had severe exacerbations of disease activity that prompted their withdrawal from the study (P = 0.06) (see Table 49-1).
Williams and associates18 in a 48-week multicenter, placebo-controlled, double-blind randomized study evaluated the efficacy and safety of HCQ in the treatment of articular complaints of 71 patients with SLE requiring less than 10 mg prednisone per day. Only joint pain favored the use of HCQ. As noted by the authors of this study, the small sample size and high dropout rate (41%) limited the power of the study (see Table 49-1).
In certain countries of Latin America and Eastern Europe, CQ is the only AM medication available. In 1996, Meinão and colleagues19 performed the first prospective, double-blind, randomized trial with CQ in 44 corticosteroid-dependent patients with SLE. At 12 months, articular involvement was present in 0% of those receiving CQ and in 67% of those receiving placebo (P = 0.001). Flares occurred more commonly in those randomized to placebo (10 patients [83%] receiving placebo, 2 [18%] patients receiving CQ). The prednisone dose was decreased in 9 patients (82%) in the CQ group versus 3 (25%) in the placebo group (P = 0.001) (see Table 49-1).
Kasitanon and others24 reported that HCQ therapy predicts complete renal remission at 12 months in patients treated with mycophenolate mofetil for membranous lupus nephritis. This study was the first to demonstrate that concurrent HCQ use has a significant benefit for a severe manifestation of SLE. If this finding is confirmed, AM medications may have a potentially new role as adjunctive therapy in other forms of severe SLE (see Table 49-1).
Wozniacka and associates5 found that CQ treatment decreased disease activity in SLE. The authors of this study also found that the levels of IL-1, IL-18, and TNF-α decreased significantly after 3 months of CQ use (see Table 49-1).
Efficacy during Pregnancy
In 2001, Levy and others21 reported the only prospective randomized, placebo, controlled study assessing the efficacy and safety of HCQ in patients with lupus who were pregnant. Twenty patients with SLE or biopsy-proven DLE were randomized to receive HCQ or placebo between 8 and 18 weeks of pregnancy. HCQ use was associated with decreased disease activity scores (P = 0.04) and lower prednisone doses at delivery (HCQ, 4.5 mg /day; placebo, 13.7 mg/day; P = 0.05). No statistically significant differences, with respect to delivery age or Apgar scores, were observed between the treatment groups. No auditory or other clinical deficits were detected. Ophthalmoscopy was normal in all patients at 12 weeks (see Table 49-1).
In a 2006 prospective study, Cortés-Hernandez and colleagues22 reported that the discontinuation of CQ significantly predicted disease flares in 103 pregnancies (see Table 49-1).
Clowse and associates23 assessed 257 pregnancies in 197 women divided into three groups: (1) no HCQ exposure during pregnancy (163 pregnancies), (2) continuous use of HCQ during pregnancy (56 pregnancies), or (3) cessation of HCQ treatment either in the 3 months before or during the first trimester of pregnancy (38 pregnancies). The rates of miscarriage, stillbirth, pregnancy loss, and congenital abnormalities were not statistically different among the three groups. SLE activity during pregnancy was significantly higher in women who discontinued the HCQ, as was the rate of disease flare among women who stopped the medication (55%), compared with those who either continued taking it (30%) and those who never took it (36%). Rates of prednisone use, as well as the prevalence of patients requiring high-dose prednisone (>20 mg/day or pulse therapy), were significantly lower in the group with continuous HCQ therapy (see Table 49-1).
No toxic or developmental delay has been reported in children whose mothers have been exposed to antimalarials during pregnancy or during lactation. Therefore maternal AM use is considered safe during pregnancy and breast feeding.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree