CHAPTER 48 Anterior Lumbar Interbody Fusion
Low back pain has long been noted to be one of the most disabling conditions in the United States and the Western world. Disability from back pain has been reported to cost approximately $100 billion annually.1 Lumbar disc degeneration is often divided into three basic categories: internal disc derangement, degenerative disc disease (DDD), and motion segment instability. Internal disc derangement2 encompasses annular tears and dark disc disease, DDD describes isolated disc resorption and spondylosis, and motion segment instability involves listhesis and scoliotic changes. This description of lumbar disc degeneration, although oversimplified, encompasses a dynamic process with overlapping findings at individual and adjacent levels. DDD, although controversial in its exact role in patients with back pain, has been shown to be a pain source generator.3,4 Removal of the pain generator, the intervertebral disc, is a viable and logical approach for selected patients.
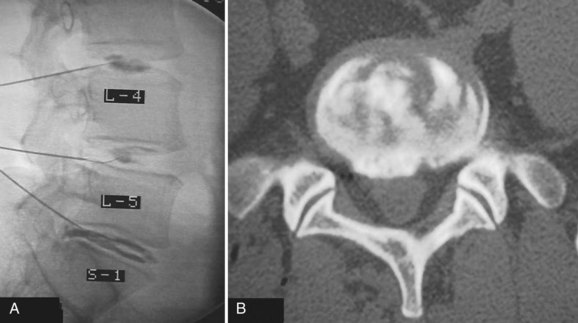
Determining the ideal candidate for surgical management of DDD can be more challenging than performing the procedure itself because of the unclear relationship between patient symptoms, diagnostic studies, and surgical outcomes. The patient should have failed conservative management, including oral medication, lifestyle modification, and active rehabilitation, before surgical intervention. To maximize the predictive value of lumbar interbody fusion for the treatment of lumbar disc degeneration, the patient’s history should be consistent with mechanical back pain, and radiographic studies should show degeneration at discrete levels. Discography, as discussed in an earlier chapter, is at this point the only diagnostic test in the authors’ opinion that can isolate discogenic pain and should reproduce concordant pain and abnormal disc morphology (Fig. 48–1). Patients with a significant behavioral component to their pain should be considered poor surgical candidates. Preoperative psychological screening can be helpful in the evaluation of these patients.5
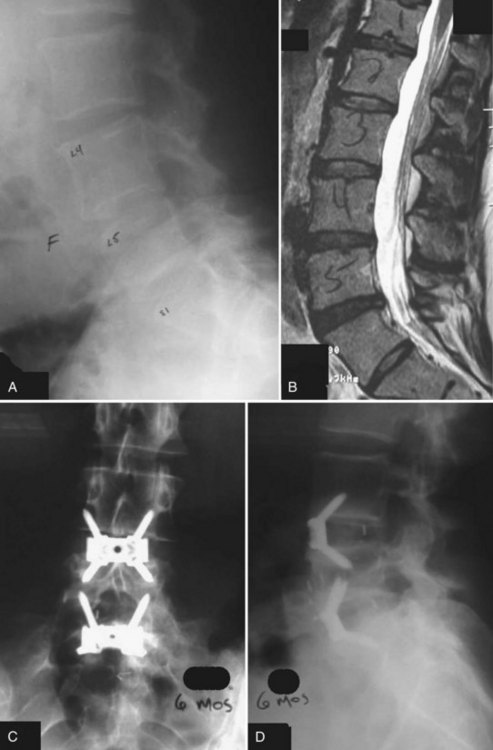
Anterior lumbar interbody fusion (ALIF), as an option for treating DDD, has had a tumultuous history with increasing and decreasing interest and success over the years. ALIF as a procedure favors load transmission through the anterior column, recreates lordosis, restores disc height, and tensions lateral and posterior annular or ligamentous fibers (Fig. 48–2).6 Direct anterior exposure allows complete removal of disc material, increasing the fusion rate,7 while avoiding trauma to the posterior musculature, making it an attractive option for the treatment of DDD.
ALIF as a treatment for low back pain was initially performed with varied success. In 1972, Stauffer and Coventry8 reported 36% good results in 77 patients with lumbar disc rupture. In 1988, Inoue and colleagues9 reviewed 350 cases of ALIF for disc herniation and found 73% of patients had relief of back pain. Blumenthal and colleagues10 reported on 34 patients in 1988 with 73% fusion rate and 74% clinically satisfactory results. Loguidice and colleagues11 found an overall fusion rate of 80% radiographically using various combinations of autograft and allograft interbody spacers. Of 85 patients, 74% responded that the surgery helped.
Anatomy and Approach
Developed in the 1990s, laparoscopic ALIF achieved early success with its pioneers.12 Laparoscopic ALIF was reported to be less invasive with less blood loss and faster recovery; however, later reports contradicted these findings, noting no identifiable advantages, added technical challenge, and increased specific complications such as retrograde ejaculation.13,14
Indications for Interbody Fusions
Axial back pain caused by the degenerative process of the disc and spine as a whole is currently the main indication for ALIF. By correctly identifying discogenic pain via radiographs, magnetic resonance imaging (MRI), and provocative discography, one can remove the pathologic disc and stabilize the motion segment with an interbody graft. Using an ALIF procedure, patients with associated radicular leg pain secondary to foraminal narrowing can be indirectly decompressed by restoring the foraminal height, elongating redundant posterior and lateral anulus (if not removed), and realigning overlapping incongruent facet joints and more directly decompressed by removing compressive nuclear material.15,16 Patients with a herniated nucleus pulposus can undergo direct decompression and discectomy via an anterior exposure. Interbody implants placed through an anterior approach can be used alone or in conjunction with posterior fusion techniques for cases requiring a more robust construct, as discussed later in this chapter.
Interbody Implants and Graft Material
The race between temporary mechanical support and biology has been run since the origins of orthopaedic care. In the spine, the goal of any interbody device is to provide anterior column mechanical support while a bony fusion develops. A single question remains to be answered: How rigid does a construct need to be to provide early stability without negatively affecting the spine when fusion is present? Creating an unnecessarily stiff construct may lead to stress shielding,17 additional surgery, and implantation of costly implants; however, too little stiffness leads to biomechanical failure or pseudarthrosis. Pilliar and colleagues18 showed that small micromotion of 28 µm does not affect bone ingrowth into porous-surfaced implants and large micromotion greater than 150 µm can produce a fibrous interface. Nevertheless, the current consensus seems to be that adequate stabilization must greatly increase the stiffness above the native segment. In vitro studies do not fully recreate in vivo experiences, and individual patient factors, such as bone quality, size, and load demand, are variable and dynamic, complicating the goal.
The first lumbar interbody fusion was reported by Burns19 in 1933, using a tibial peg to treat spondylolisthesis. Interbody fusion for DDD was described by Harmon20 in 1963 and then by Crock2 and Stauffer and Coventry.8 Early days of interbody grafting incorporated the use of bicortical or tricortical spacers harvested from iliac crest. When used alone, this graft is associated with significant rates of mechanical failure, loss of correction, and pseudarthrosis.21,22 Sterile allograft (i.e., bone dowels or tricortical grafts) have often been used because they are stronger than equivalent fresh, autologous bone and eliminate the need for autologous harvesting.23
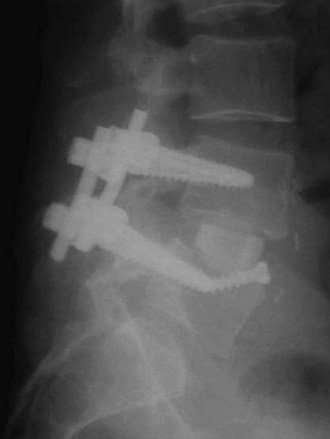
Femoral ring allograft (FRA), used frequently, obviates the need for cortical autograft and provides a strut with significant compressive strength,22,23 incorporates with host bone, and provides a medium easier than metal to evaluate graft incorporation (Fig. 48–3). Previously, allograft rings were fashioned by surgeons on back operating room tables. Now precision machined grafts provide surgeons the option to trial size and implant the most appropriately sized graft to fit the patient’s anatomic needs and obtain the most stable construct with the allograft reaching the dense peripheral ring of subchondral bone. To augment fusion, allograft or autograft cancellous bone can be placed in the center of the cortical ring. FRA as a stand-alone intervertebral spacer has been shown to have a high rate of pseudarthrosis and subsidence.24,25 Anterior or posterior augmentation has been recommended.
Holte and colleagues26 reported in 1994 on the use of FRA with and without supplemental posterior fixation. These investigators achieved fusion rates of 96% in some cases, depending on the number of levels fused. Sarwat and colleagues27 reviewed 43 patients undergoing combined anterior-posterior fusions using FRA packed with cancellous allograft with supplemental posterior fixation; 100% of the one-level fusions and 93% of the two-level fusions were radiographically deemed solid fusions.
The addition of posterior fusion, instrumentation, and cortically lined grafts added to the mechanical stability of these constructs, but surgical time and complications led surgeons to seek other options. Transforaminal lumbar interbody fusion and posterior lumbar interbody fusion allowed surgeons to place interbody grafts from posterior approaches, providing anterior column support and negating anterior exposures. Posterior based surgery has been shown, however, to have increased operative time, patient morbidity, and complications compared with anterior based surgery.28,29 Also, patient anatomy and the degree of pathology can make achieving an adequate discectomy and ideal graft size and position and placement a challenge. These factors have contributed to less restoration of disc height, less than complete fusion bed preparation, and less mechanical stability at the time of implantation than with ALIF.
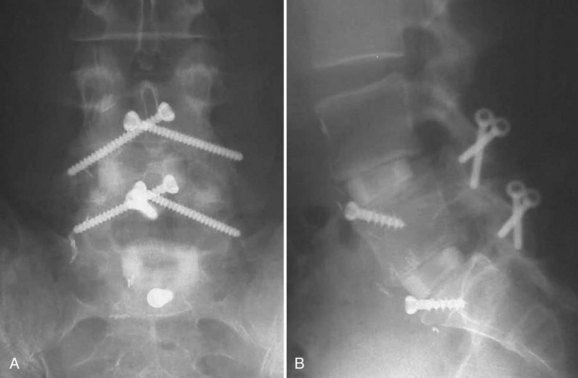
Horizontal threaded cylinder cages, typically made from titanium, were brought on the market with huge fanfare but have fallen out of favor. These were originally developed to treat wobbler syndrome, which is a chronic cervical instability causing myelopathy in thoroughbred horses.30 The U.S. Food and Drug Administration (FDA) investigational device exemption (IDE) studies for the BAK (Zimmer, Warsaw, IN) and Ray TFC (Stryker, Kalamazoo, MI) indicated that threaded cages used as stand-alone devices without supplemental posterior fixation performed well (Fig. 48–4).31,32 Ray32 reported his results during the FDA IDE clinical trial using the Ray titanium fusion cages in 236 cases showing 91% fusion rate and 80% average clinical improvement.
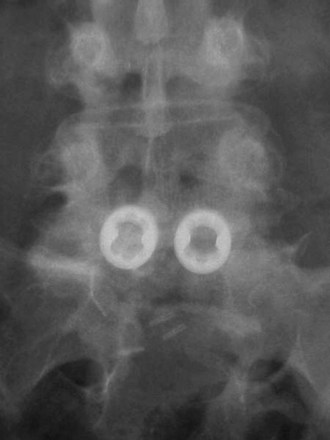
FIGURE 48–4 Anteroposterior radiograph of threaded fusion cages at 1 year, with bone growth across cages.
Kuslich and colleagues31 reported on 247 ALIF patients with 24-month follow-up; they observed a fusion rate of 98% among single-level procedures and 80% among two-level procedures. They reported clinical pain improvement from a mean of 5 to 2.9 at 2 years, on a scale of 0 to 6. The functional scores improved from 20.9 preoperative mean to 15.2 at 2-year follow-up, on a 7 to 32 point scale evaluating sitting, walking, and other activities of daily living and recreational activities. Tran and colleagues33 reported that only 5.2% of their group underwent additional posterior surgery at the same level for unresolved or new-onset pain, supporting the use of stand-alone cages. Preparation of the disc space for threaded cages violates the peripheral subchondral ring of bone, theoretically increasing the risk of subsidence. Although approved for stand-alone interbody fusion, threaded cages were later questioned for their amount of resistance to motion in the unstable spine; some authors adopted a 360-degree approach.34,35
The advent of more stable anterior column support allowed surgeons to use less invasive posterior surgical options with stronger biomechanical constructs than with an associated posterolateral fusion.6 Translaminar screws, facet screws, and percutaneous pedicle screw constructs (termed 270-degree fusions) provided surgeons the additive effect of immediate mechanical strength and long-term fusion stability without necessitating significant posterior fusion bed creation, associated soft tissue devitalization, and patient morbidity (Fig. 48–5).36–38 Ferrara and colleagues39 compared pedicle and translaminar facet screws and found both to be reliable constructs with similar properties after 180,000 cycles and recommended that surgeon preference and patient-specific needs could drive the choice. In 2001, Schofferman and colleagues40 prospectively compared the addition of posterior spinal fusion in patients who had ALIF with pedicle screw instrumentation (360 degrees vs. 270 degrees). These investigators found that posterolateral fusion was associated with greater blood loss, operating room time, and cost, with no significant improvement in the arthrodesis rate; in addition, 68% of posterolateral fusions failed to show radiographic fusion.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree