18 ANALGESIA, SEDATION, AND NEUROMUSCULAR BLOCKADE IN THE TRAUMA PATIENT
INTRODUCTION
Pain management in the adult trauma patient is multifactorial and complex, which presents unique challenges to nursing care. Traumatic injuries are the consequence of external forces, and subsequent delayed sequelae are multifaceted and painful. Throughout the various stages of the trauma care continuum that follows blunt or penetrating injuries, a patient may require a balanced regimen of analgesia, sedation, and possibly neuromuscular blockade (NMB). It is imperative that health care practitioners apply evidence-based practice principles to the management of an injured patient. This chapter reviews current consensus guidelines and multimodal approaches to individualize quality patient care through the phases of resuscitation to rehabilitation.
NEGATIVE CONSEQUENCES OF UNDERTREATED PAIN
Unabated acute pain creates negative consequences and can lead to persistent pain.1 Physiological, psychosocial, and economic parameters can be affected throughout the trauma continuum. Table 18-1 summarizes the consequences of inadequately controlled pain. Despite high technologic advances in analgesic delivery, negative outcomes of atelectasis, hypoxemia, pneumonia, deep vein thrombosis, and ileus continue to prevent successful recovery after surgery and traumatic injury. Anxiety, fear, and depression are likely to occur if pain is persistent and goes untreated. The economic impact of undertreated pain can result in prolonged hospital stay and continued health care resource utilization on discharge.2
TABLE 18-1 Consequences of Uncontrolled Pain: A Systems Overview
| System | Response | Outcome |
|---|---|---|
| Neurologic | ↓Mental function, ↓cognitive function | Anxiety, fear, anger, depression, confusion |
| Respiratory | ↓Vital capacity, ↓functional residual capacity, ↓tidal volume, ↓cough effort, ↓thoracic and abdominal muscle movement, ↓alveolar ventilation, ↓oxygen saturation | Atelectasis, pneumonia, hypoxemia |
| Cardiovascular | ↓Heart rate, ↓cardiac output, ↓peripheral vascular resistance, hypercoagulation, ↓systemic vascular resistance, hypertension, ↓coronary vascular resistance, ↓myocardial oxygen consumption | Angina, deep vein thrombosis |
| Gastrointestinal | ↓Gastric and bowel motility | Ileus, nausea and vomiting, constipation |
| Genitourinary | ↓Urinary output, ↓urinary sphincter tone | Urinary retention |
| Musculoskeletal | Impaired muscle function | Weakness, muscle spasm, fatigue |
| Metabolic/endocrine | Hyperglycemia, gluconeogenesis, glucose intolerance, insulin resistance, ↓ACTH, ↑cortisol, ↑ADH, hepinephrine, ↑norepinephrine, ↑growth hormone, ↑catecholamines, ↑renin, ↑angiotension II, ↑aldosterone | Weight loss, fever, tachycardia |
ACTH, Adrenocorticotropin; ADH, antidiuretic hormone. Adapted from McCaffery M, Pasero C: Pain: clinical manual, St Louis, 1999, Mosby, p 24, Joshi GP, Ogunnaike BO: Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain, Anesthesiol Clin North Am 23:21-36, 2005.
Undertreated pain has been well documented in the literature, spanning more than 30 years.3 The term “oligoanalgesia” has evolved in the emergency medicine arena to demonstrate the practice of undermedication in patients who report pain as their chief complaint. Health care practitioners in the past were known to withhold analgesics to prevent masking of clinical findings despite the report of pain. Puntillo et al4 reported clinically significant differences between nursing and patient pain rating scores, documenting that emergency department nurses underestimated musculoskeletal pain 95% of the time. Emergency department studies showed that 53% of patients admitted waited more than 60 minutes before analgesics were administered and those reporting a mild pain score of less than 4 (out of 10) received no analgesics.5 Although prehospital protocols have been established for first responders to administer analgesics in the field, in one study of analgesic administration in more than 1,000 patients, only 18 (2%) were given morphine or nitrous oxide for suspected extremity fracture.6 Young children under 6 years of age, individuals with cognitive impairment, the elderly, female patients, ethnic minorities, and those with psychiatric illness are at the greatest risk for undermedication during an emergency department admission.7–9 At a Level I trauma center, patients at risk for oligoanalgesia were identified as those more seriously injured, as defined by a lower Revised Trauma Score, a lower Glasgow Coma Scale score, and those who were intubated.10
Many factors contribute to the underuse of analgesics during various points of care when a patient seeks medical attention for pain relief from injury or disease. Lack of knowledge regarding pharmacologic management appears to be a common theme among many disciplines.11 Some care providers want to make a diagnosis before administering analgesics.12 Lack of consistent assessment of pain is another factor that affects analgesic administration, especially when pain severity scores are not documented in the medical record. Those patients unable to communicate pain, such as the very young, the unconscious, and the elderly, usually do not receive analgesics.7 Another barrier is the belief among emergency care physicians that a valid, informed consent needs to be obtained before administration of opioids, to prevent interfering with the patient’s decision-making capability.3,13
Undertreatment of pain may be a risk in the critical care environment, especially when vital organ function becomes the priority. The administration of analgesics is limited during events of hemodynamic instability or in the presence of deteriorating mental status with head injury. Assessment of pain in the critically ill is limited as a result of the lack of self-report, especially if the patient is intubated and sedated.14 Patients unable to report pain are at risk for undermedication, especially if pain behaviors such as facial expression or body movement are absent because of paralysis or because of administration of sedatives and paralytics.15
In 1992 the Agency for Health Care Policy and Research (AHCPR), which is now the Agency for Healthcare Research and Quality, published the first clinical practice guideline (CPG) on pain management in operative or medical procedures and trauma.16 This publication was the springboard for the development of subsequent CPGs that address other pain syndromes and conditions. The Joint Commission (TJC) applied pain management assessment and management requirements in many practice arenas in 2001. Both these organizations have been instrumental in the formation of a standardized practice of pain assessment and delivery of quality pain management. Undertreatment of acute pain can lead to the development of chronic pain states or syndromes.17,18
DEFINITIONS/CLASSIFICATIONS/PHYSIOLOGIC PATHWAYS
DEFINITIONS
More than 35 years ago, McCaffery defined pain as “whatever the experiencing person says it is, existing whenever she/he says it does.” This exemplifies the importance of the patient’s perspective and input, which supports the individual’s self-report as the most reliable indicator of pain.16 The most widely recognized definition of pain, “an unpleasant sensory and emotional experience associated with actual or potential damage,” was introduced by the International Association for the Study of Pain (IASP) in 1979.19 It is a sensation that is strictly subjective in nature. Pain is a very complex, individualized experience with many dimensions.
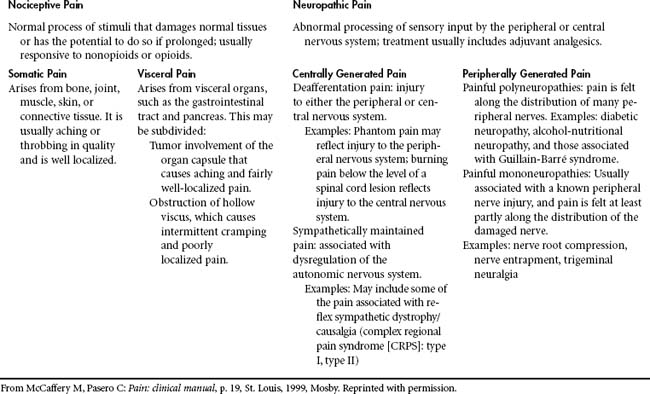
Pain experienced during the various stages of the trauma continuum can be acute, intermittent or procedural, chronic (persistent), or neuropathic in nature (Table 18-2). Acute pain can be considered short in duration, as a result of direct tissue trauma. The perception of discomfort declines as tissue inflammation diminishes and healing occurs over time. Intermittent or procedural pain can be the result of routine care interventions, such as venous puncture, dressing changes, or suctioning. Turning has been recognized as one of the most painful and intermittent distressing events endured by patients in the intensive care unit (ICU).20 Chronic pain is acquired from long-standing processes of tissue injury or unrelieved pain that results from tissue hypersensitity. Neuropathic pain can be derived from damage or dysfunction in the peripheral or central nervous system (CNS) that displays sensory symptoms and signs21 (Table 18-3). The trauma patient can also have acute pain in addition to coexisting pain syndromes.
| Type of Pain | Characteristics |
|---|---|
| Acute pain | Warning signal for potential of injury (protective reflex); complex, individualized experience; nociceptive in nature; usually some degree of tissue damage; hyperdynamic response related to hormone release; pain experience declines with tissue healing; may convert to chronic or neuropathic pain |
| Intermittent pain | Nociceptive in nature; predictable or unpredictable; pain intensity experience increases as stimulus exposure is prolonged; procedural pain (venipuncture, dressing changes, movement); “breakthrough” pain or end of dose pain from scheduled analgesic; procedural pain can induce suffering perception/anxiety |
| Chronic (persistent) pain | Persistent pain extending beyond a period of healing; disrupts activities of daily living and sleep; interrupts functional status; no adaptive or physiologic response; may be nociceptive or neuropathic or both; environmental and affective behaviors may exacerbate or perpetuate |
| Cancer pain | Malignant pain; acute and chronic components; pathology and level of pain are related |
| Chronic noncancer pain | Subtype of chronic pain; acute injury related or no discernible cause; prolonged, possibly life-long pain; affects daily activities, relationships, physical deconditioning; psychologic symptoms develop |
Adapted from Turk DC, Okifuji A: Pain terms and taxonomies of pain. In Loeser JD et al, editors: Bonica’s management of pain, 3rd ed, pp. 17-25, Baltimore, 2001, Lippincott Williams & Wilkins.
PAIN CLASSIFICATIONS
One way to classify pain is by inferred pathophysiologic mechanisms22 (Table 18-3). Nociception refers to the body’s response and processing to a noxious (or painful) stimulus along an ascending pathway. The peripheral nervous system contains free nerve endings, called nociceptors, that are responsible for pain. These receptors transmit the sensation of painful stimuli from peripheral tissues that have been injured to the cerebral cortex. Nociceptors are located in skin, joints, muscle, fascia, viscera, and the smooth muscle of the arterial walls. Two types of afferent nerve fibers or first-order neurons, A-delta and C-fibers, transmit pain through neural pathways. These fibers are located in the dorsal horn of the spinal cord (Figure 18-1). A-delta fibers are large diameter and myelinated fibers that transmit fast-moving impulses caused by thermal and mechanical stimuli. Pain sensations can be described as sharp and sudden. C-fibers are small diameter and unmyelinated fibers that transmit delayed, slow pain that has been activated by chemicals (neurotransmitters) released after cellular trauma. These neurotransmitters include histamine, serotonin, and acetylcholine. Pain descriptions verbalized by the patient include dull, diffuse, and prolonged.
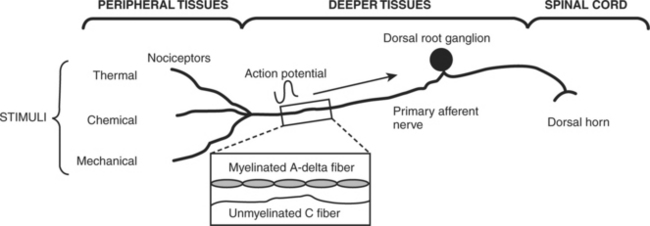
FIGURE 18-1 The physiology and processing of pain.
(Redrawn from Renn CL, Dorsey SG: The physiology and processing of pain: a review, AACN Clin Issues 16:277-290; quiz 413-415, 2005.)
Neuropathic pain arises from abnormal processing of a sensory stimulus by the peripheral nervous system or the CNS.22 The N-methyl-D-aspartate (NMDA) receptor is believed to be involved in neuropathetic and chronic pain states.23 Nerve injury can also activate changes in the CNS and result in central sensitization and “wind-up.” Wind-up or hyperexcitibility occurs at the dorsal horn level as an outcome of persistent noxious stimuli.22 Peripheral sensitization occurs related to the endogenous chemical release and inflammation that follows. Characteristics of neuropathic pain include hyperalagesia, burning, tingling, and electric-shock pain perception.
PHYSIOLOGIC PATHWAYS
There are four processes in the sensory pathway, which include transduction, transmission, perception, and modulation (Figure 18-2). Within each of these processes, analgesic therapy can be targeted to attenuate the pain experience. Transduction involves the process of converting chemical, mechanical, or thermal energy into neural impulses. In the presence of trauma or infection, chemical substances and enzymes are released from the damaged tissues, increasing the transduction of painful stimuli. Byproducts of the arachidonic acid pathway, which include prostaglandins (PG) and leukotrienes, are the major mediators of hyperanalgesia with inflammation. Histamine (H) is released from mast cells and acts directly on sensory neurons to produce itching and pain. Other sensitizing chemicals include bradykinin, serotonin (5HT), and substance P (SP). The pain stimulus causes an action potential where ion transfer occurs before impulse conduction.
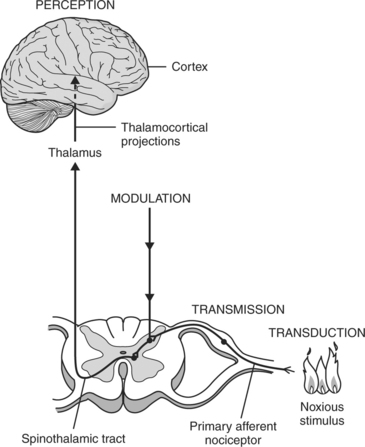
FIGURE 18-2 Four processes in sensory pathway: transduction, transmission, perception, and modulation.
(From Ferrante and VadeBoncouer, editors: Postoperative pain management, New York, 1993, Churchill-Livingstone.)
The CNS has two mechanisms that are part of pain transmission: the ascending pathway through conduction and the descending pathway through modulation (Figure 18-3). Pain receptors may be stimulated by temperature extremes, mechanical injury, or chemical irritation. The ascending tracts synapse with the first-order A-delta and C-fibers located in laminae I through V of the spinal cord. Type C fibers are mainly found in lamina I and II, known as the substantia gelatinosa, and synapse with neurons in the dorsal horn. The second-order neurons, arising from the dorsal horn, travel cephalad to the thalamus.
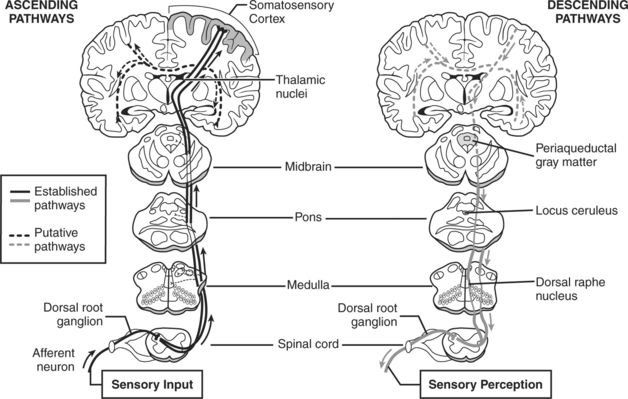
FIGURE 18-3 Normal processing of acute nociceptive pain.
(Redrawn from Hainline B: Chronic pain: physiological, diagnostic, and management considerations [review]. Psychiatr Clin North Am 28:713-735, 2005.)
Several pathways for afferent pain signals traverse the ascending spinal cord to the cortex. The spinothalamic pathway is regarded as the most important pathway, and it can be subdivided into the neospinothalamic (lateral spinothalamic) pathway and the paleospinothalamic (medial spinothalamic) pathway.24 The neospinothalamic is the primary pathway for sensations of pain, temperature, and touch initiated by fast pain signals.25 The paleospinothalamic pathway is principally for the transmission of unmyelinated type C fibers that are conductive of slow or chronic pain sensations.26 SP is believed to be the main neurotransmitter of the paleospinothalamic pathway in the dorsal horn.24 The spinomesencephalic pathway plays a role in the activation of descending control systems and endogenous opioid systems. It begins with the nociceptors in laminae I and V and terminates in the roof of the midbrain and the periaqueductal gray matter.27
Cortical understanding of the noxious stimulus occurs at the somatosensory cortex, where perception of pain is processed and individualized. The spinothalamic tract moves through the pons, medulla, and midbrain and terminates in the thalamus. Pain location, intensity, and emotional processing occur at this level. The reticular system is responsible for the autonomic response to pain and the emotional-affective components.28 The somatosensory cortex localizes and characterizes pain. The limbic system is responsible for emotional processing and the behavioral reaction to pain.22
The descending pain pathway is the endogenous pain modulation system. Modulation involves the modification of nociceptive transmissions in the dorsal horn or spinal reflexes. Neurons originating from the brainstem descend to the dorsal horn, releasing endogenous opioids, (5HT, norepinephrine, and γ-aminobutyric acid (GABA), which inhibit the transmission of noxious stimuli and produce analgesia. Three subtypes of endogenous opioids include β-endorphin, met-and leu-enkephalins, and dynorphins.24,29,30 Endogenous and exogenous opioids activate dorsal horn receptors mu (μ), delta (δ), and kappa (κ) to inhibit pain impulses.22
PAIN ASSESSMENT IN THE ADULT TRAUMA PATIENT
Pain assessment in the acutely injured is a clinician’s challenge in a fast-paced environment of rapid response and resuscitation. Pain assessment must be individualized, and basic principles of a comprehensive analgesic plan can be applied using the ABC method as described by the AHCPR (Table 18-4).31 Instituting pain protocols after the primary survey and a pain assessment may be an opportunity to facilitate analgesic administration for traumatic injuries and during pain-provoking activities.32
TABLE 18-4 Pain Assessment and Management Mnemonic
| A | Ask about and Assess pain regularly and systematically. |
| B | Believe the report of pain. |
| C | Choose the best pain control options. |
| D | Deliver pain interventions consistently and timely. |
| E | Empower the patient and family to control the course. |
Modified from Jacox A, Carr DB, Payne R et al: Management of cancer pain, Clinical Practice Guideline No. 9, AHCPR Pub. No. 94-0592, Rockville, 1994, Agency for Health Care Policy and Research, U.S. Department of Health and Human Services, Public Health Service.
A comprehensive approach to trauma pain assessment requires the careful evaluation of key elements (Table 18-5). Although the self-report of pain is the gold standard, the unconscious, the cognitively impaired, the young and the old, the intoxicated, and those with communication or language barriers offer unique assessment challenges to the trauma team. A systematic pain assessment should include a general patient health history, pain history, a physical examination with behavioral observations, and further diagnostic investigation as needed. When the self-report cannot be articulated, other measures must be used to detect the presence of pain.
TABLE 18-5 Pain Assessment Factors in the Critical, Intermediate, and Rehabilitative Phases of Trauma
The patient history should explore the medical history, the presence of comorbities, and the mechanism of injury. With regard to previous hospitalizations, previous surgical interventions should be detailed, along with analgesic methods and agents that were effective in the past. In addition to the patient’s medical history, medication reconciliation will validate current analgesic exposure and alert the health care team to anticipate the response to analgesic treatments chosen. A pain history should include information regarding pain medication allergies or sensitivities and further investigation is warranted if previous adverse reactions are mentioned. Current use of analgesics, whether over-the-counter products or prescription analgesics and sedatives, should be validated and current frequency of administration investigated. Smoking, alcohol consumption patterns, and history of substance abuse and detoxification require further elaboration with regard to patterns of use and duration. Cultural and religious beliefs and emotional response to past pain experiences should also be explored.
A history of chronic pain syndromes, previous pain treatments, and psychosocial responses to pain and stress should be interconnected to the initial pain assessment. Patient personality and expectations concerning pain and its management must be considered. Identifying individual coping responses, pain behaviors, and cultural interpretations are important. Patient responses to painful situations can be obtained from the patient or validated by a family informant. Establishing a comfort goal33 or acceptable pain rating should also be included during the initial pain screen if possible.
The provoking aspects and causes of pain must be investigated thoroughly. Inquiries should include what triggers the pain (such as movement or deep breathing), specific treatments and procedures (such as wound dressing changes), and its duration: is the pain constant or episodic? Does tactile stimulation, such as pressure from orthopedic appliances or bed linens, cause discomfort? Palliative measures may include analgesic administration, elevation, realignment, and support of the injured body part.
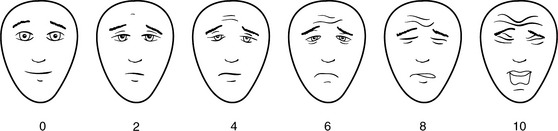
Pain rating scales offer a quick and simplified means for a patient to rate pain severity. Consistently using the same rating tool is imperative throughout the trauma continuum for assessment and documentation. Verbal rating intensity scales (Figure 18-4) aid the practitioner evaluating traumatic pain complaints. Unidimensional scales, such as the numeric scale (where 0 is no pain and 10 is the worst pain imaginable), word descriptor scales (no pain, mild, moderate, severe), or visual scales (Faces Pain Scale-R) allow the clinician to determine the patient’s pain intensity within a short time frame. Many tools are available, with some using horizontal or vertical linear orientation or variations of color. The Bieri FACES-R pain rating scale can be used in patients older than 3 years of age and those with cognitive impairment (Figure 18-5). Multidimensional tools, such as the McGill Pain Questionnaire, provide in-depth pain information and effects on daily living and require a motivated patient to complete. These instruments are more helpful during the intermittent and rehabilitative phase of care when complex, persistent pain may require specialized care and follow-up.
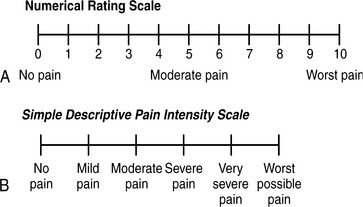
FIGURE 18-4 Verbal rating intensity scales. A, Numerical rating scale.
(Redrawn from McCaffery M, Beebe A: Pain: clinical manual for nursing practice, 2nd ed, Philadelphia, 1999, Mosby.) B, Simple descriptive pain intensity scale. (From Acute pain management: Operative or medical procedures and trauma, Clinical Practice Guideline No. 1, AHCPR Pub. No 92-0032, Rockville, Md, Feb 1992, Agency for Healthcare Research and Quality, pp 116-117.)
For the unconscious, sedated, or nonverbal patient, accurate pain assessment is difficult and results in underrated and undertreated pain.34,35 These patients are unable to verbalize a self-report of pain. Pain in this patient population can be perceived as suffering, which presents a distinctive set of behaviors perceived by caregivers.36 Behavioral manifestations such as restlessness, moaning, crying, or guarding the injured body part can cue the nurse to the presence of pain or discomfort. Other behaviors include teeth clenching, forehead wrinkling, restlessness, withdrawal reflexes, thrashing, rocking, kicking, and tensing muscles.37
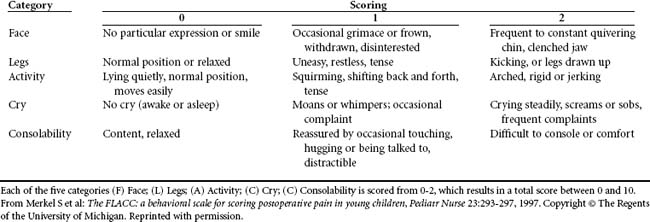
Behavioral assessment tools have been frequently used in the pediatric population, with specific tools developed for certain patient age groups and diagnoses (Table 18-6). Historically, nurses relied on physiologic indicators such as increased heart rate or blood pressure, which are now considered the least sensitive indicators of pain.38 With behavioral indicators, the nurse must assume that pain is present15 and provide nursing interventions, such as repositioning, and offer an analgesic trial.
A behavioral tool has been validated in the critically ill, sedated, and mechanically ventilated patient population35,39 (Table 18-7). It scores facial expression, movement of upper limbs, and ventilator compliance. Other pain-related behavioral checklists available include domains of agitation, anxiety, ventilator synchrony, facial tension, muscle tone, body movement, vocalizations, and consolability.40–42 Adoption of a single behavior tool exclusively for the trauma patient is limited because of many variables, especially when the patient requires tracheostomy and mechanical ventilation. For the behavior scale to be valid, the patient must be able to score in all behavior categories.15 Vocalization or expressions of pain cannot be verbalized, especially when concurrent sedation or NMB is administered. Diaphoresis and lacrimation have been observed in patients receiving NMB during painful stimulation.43 Patients who are undergoing procedures that would be painful for others should be treated pre-emptively for pain.34 Use of a sedation assessment tool, such as the Ramsay Sedation Rating Scale, the Richmond Agitation-Sedation Scale (RASS), or the Motor Activity Assessment Scale (MASS) is an appropriate alternative, as long as pain is assumed present with analgesics administered. Discussion of these tools occurs later in the chapter.
TABLE 18-7 Behavior Pain Scale*
| Item | Behavior | Score |
|---|---|---|
| Facial expression | Relaxed | 1 |
| Partially tightened (brow lowering) | 2 | |
| Fully tightened (eyelid closing) | 3 | |
| Grimacing | 4 | |
| Upper limb movements | No movement | 1 |
| Partially bent | 2 | |
| Fully bent with finger flexion | 3 | |
| Permanently retracted | 4 | |
| Compliance with mechanical ventilator | Tolerating movement | 1 |
| Coughing but tolerating ventilation for most of the time | 2 | |
| Fighting ventilator | 3 | |
| Unable to control ventilation | 4 |
* The Behavior Pain Scale allows the assessor to derive a score of between 3 (no pain) and 12 (highest pain score).
From Payen J-F, Bru O, Bosson J-L, et al: Assessing pain in critically ill sedated patients by using a behavioral pain scale, Crit Care Med 29:2258-2263, 2001.
For the patient who is cognitively impaired, perhaps from the aging process or brain injury, the self-report of pain can be elicited through the use of standardized pain rating scales.44,45 It is important for the nurse to determine whether the patient is able to provide a self-report and to modify the approach for assessment on the basis of the patient’s cognition. Simplifying the descriptive anchors on the intensity scale may be required, as well as using another scale to validate the patient’s response. Use of words such as mild, moderate, and severe, with explanation of their meanings on a number scale is one strategy. If the verbal descriptor scale is inclusive, validation with a visually enlarged Faces Pain Scale is an alternative.46 Behavioral patterns can also be incorporated into the pain assessment, especially with movement. Baseline behavior and activity patterns should be documented so that changes can be further investigated.46 Behavioral tools can also be applied for those patients unable to articulate a pain rating.47
Assessment of pain has been endorsed as the fifth vital sign48 and should be adopted as a standing routine for any point-of-care documentation throughout the trauma continuum. This approach to assessment is useful in making pain assessment and treatment a priority. Frequency of assessment is directly related to its presence and severity. Current pain intensity, worst pain experienced, level of pain relief achieved, and acceptable and not acceptable pain ranges should be incorporated within the patient’s plan of care. If the intensity rating is high, pain assessment should be performed more frequently, as often as every 2 hours or more as needed to address the patient’s report of pain effectively. As pain is controlled and becomes less acute, the frequency of assessment can decrease. It is imperative to explore new reports of pain and the effectiveness of analgesic therapeutics, at rest and with movement.
TJC has developed standards for assessment and reassessment of pain.49,50 Because pain should be assessed in all patients, the clinical documentation process should be institution specific and should incorporate key elements within the permanent record. Quality indicators and electronic prompts within the information system can facilitate compliance with practitioner documentation. Consistency with recording the pain rating is important. If the numeric scoring system is used, the patient rating over the range is suggested. For example, if a patient reports a pain rating of 5 on the 0 to 10 scale, nursing documentation would be “5/10.” For those patients unable to verbalize a pain rating, a behavior tool can be used for documentation trends. It is imperative for clinicians to understand that a pain behavior score does not equate to a pain score rating.15 The pain rating, along with medication recording, can track trends with effectiveness during reassessment. Re-evaluation of analgesic effectiveness is recommended within 15 to 30 minutes of parental administration and within 1 hour of oral administration.16
OPIOIDS
EFFECTS OF OPIOIDS
Three major classes of opioid receptors have been clinically relevant for analgesia: mu (μ), delta (δ), and kappa (κ).51 Other receptors have been identified, including varespsilon, zeta, iota, sigma, and lambda.52,53 Mu opioid receptors are found in the periphery after inflammation, the dorsal horn of the spinal cord, the brainstem, the thalamus, and the cortex.54 At the cellular level, opioids decrease calcium ion entry, thus blocking SP release from the primary afferents in the dorsal horn of the spinal cord.55 Mu-1 receptors have been linked to mediate supraspinal and spinal analgesia, whereas mu-2 receptors are responsible for respiratory depression and decreased gastrointestinal motility.51 The delta receptors are responsible for spinal and supraspinal analgesia; kappa receptors are responsible for spinal analgesia and psychometric effects (dysphoria, sedation, miosis, respiratory depression).52
Opioids produce their major effects on the central nervous and gastrointestinal systems. Some of these effects are analgesia, sedation, mood changes, pupillary constriction, respiratory depression, decreased intestinal motility, nausea, and vomiting. Opioids produce a dose-dependent reduction in the responsiveness of the brainstem respiratory center, making it less responsive to increases in carbon dioxide retention. This leads to a reduction in the rate of breathing, prolonged pauses between breaths, delayed exhalation, and periodic breathing.56 Sedation, drowsiness, and mental clouding can be potentiated when opioids are administered concurrently with antianxiety and sedative-hypnotic agents. Opioids have no significant amnestic or anxiolytic properties; therefore, coadministration of a benzodiazepine or propofol is necessary to achieve controlled levels of sedation and amnesia when patients require conscious sedation or prolonged mechanical ventilation.
TYPES OF OPIOIDS AND OTHER ANALGESIC AGENTS
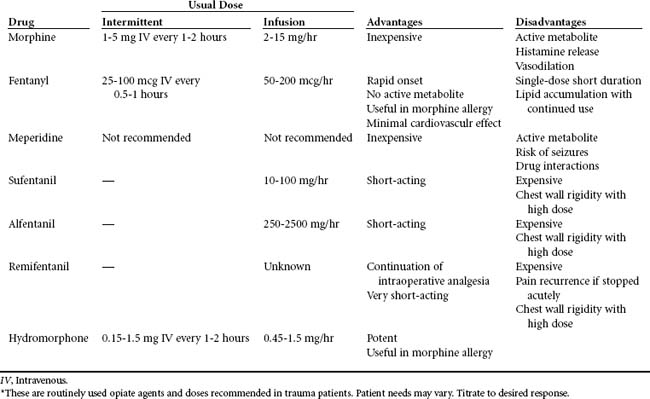
See Table 18-8 for a summary of opioids.
Morphine
Morphine is the most commonly used opiate agent and the standard of comparison for other analgesic agents. It has a plasma half-life range of 2 to 3 hours with a 4- to 6-hour duration of analgesia. Peak effect after a single intravenous bolus of morphine occurs within 15 to 30 minutes,34 and the duration of clinical effects is 2 to 7 hours. Morphine is hydrophilic, a property that results in a slower onset of analgesia. When it is administered epidurally, its onset is between 30 and 60 minutes.57 It is metabolized by the liver to water-soluble glucuronides. Morphine-3-glucuronide has no analgesic activity and is readily excreted by the kidney. Morphine-6-glucuronide (M6G) is an active metabolite that produces potent opioid effects.58 In patients with renal impairment, M6G accumulates and can result in persistent sedation and respiratory depression after discontinuation of morphine.
Meperidine
Meperidine is less potent and has a shorter half-life than morphine. Clinically, meperidine is not recommended for more than 48 hours because of the active metabolite normeperidine, which produces neurotoxicity, clinically evidenced by anxiety, tremors, myoclonus, and generalized seizures.34 Normeperidine is excreted in the urine and accumulates in the elderly or patients with renal insufficiency after doses of 600 mg per 24 hours.34 Serotonin syndrome resulting from reactions with monoamine oxidase inhibitors, tricyclic antidepressants (TCAs), or selective serotonin reuptake inhibitor (SSRI) antidepressants has been implicated with concurrent use of meperidine.59 This is an excitation syndrome characterized by hypertension, hyperpyrexia, muscle rigidity, and changes in level of consciousness.
Fentanyl
Fentanyl is a semisynthetic, lipophilic opioid, 80 to 100 times as potent as morphine.29 By the intravenous route, it has a rapid onset of action (1-5 minutes) and a short duration of action (about 1 hour).57 Onset of analgesia after intraspinal administration occurs in 5 to 15 minutes.22 It is metabolized by the liver and undergoes substantial biotransformation.60 The elimination half-life ranges from 3 to 12 hours and is affected by storage in fatty tissue, especially with prolonged administration.61 Norfentanyl, the primary metabolite, is inactive.62 Intermittent doses of fentanyl are used for procedures requiring conscious sedation. It can be administered as a continuous infusion for sustained effects in monitored settings. In comparison with morphine, fentanyl does not induce H release and produces minimal cardiovascular effects.57 However, hypotension can still occur in the volume-depleted patient. Slow incremental titration is recommended because of reports of bradycardia, severe respiratory depression, and chest wall rigidity with rapid, high-dose intravenous administration.57
Sufentanil, Alfentanil, and Remifentanil
These fentanyl analogs have been used in trauma patients, but higher cost has limited their use. Sufentanil is approximately 10 times more potent than fentanyl.63 It is highly lipophilic and has a faster onset and shorter duration of action than fentanyl. By the intravenous route, it has a rapid onset of action (1-3 minutes), with a peak at 8 to 15 minutes.64 Through the epidural route, peak action is seen in 20 minutes.65 It is metabolized by the liver and small intestines and has minimal cardiovascular effects.66
Alfentanil is superior to fentanyl and sufentanil and is one fourth as potent as fentanyl.67 Its onset of action (within 2 minutes) and duration of action (10 minutes) are shorter than those of fentanyl.67 Alfentanil offers intense analgesia and is highly useful for potentially painful procedures such as dressing changes.68
Remifentanil is an ultra-short-acting fentanyl congener, with a potency roughly 20 to 30 times that of alfentanil.69 Its onset of action is 1 to 3 minutes, with a duration of 3 to 10 minutes.70 Unlike other opioids, the liver does not metabolize remifentanil. Instead, it is rapidly hydrolyzed by plasma and tissue esterases.71 This ultra-short-acting agent (half-life of 3 minutes) is expected to produce tolerance more rapidly; thus, its use is limited in the ICU environment.72
Hydromorphone
Hydromorphone is a potent, hydrophilic synthetic derivative of morphine that produces less sedation, pruritus, nausea, and vomiting than do equivalent doses of morphine. It is five to ten times more potent than morphine and can be given orally, parenterally, or neuraxially. The onset of analgesia after epidural administration is 15 to 30 minutes.22 Hydromorphone offers minimal hemodynamic effects and no H release. It may cause a cross-reaction in morphine-allergic patients. It is the third-line agent recommended by the Society of Critical Care Medicine (SCCM) for use in hemodynamically unstable patients or patients with renal impairment because it produces no active metabolites.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree