Chapter 142 Advances in Anticoagulation for Total Joint Arthroplasty—The Newer Agents
In the United States, the number of total hip arthroplasties (THAs) is expected to increase by 174% to 572,000, and total knee arthroplasties (TKAs) by 673% to 3.48 million by 2030.29 Patients undergoing THA or TKA are at risk of developing venous thromboembolism (VTE),14 which comprises deep vein thrombosis and (DVT) and pulmonary embolism (PE). Without thromboprophylaxis, 42% to 57% of patients undergoing THA have venographically confirmed total (proximal or distal) DVT.14 Among patients undergoing TKA without thromboprophylaxis, 41% to 85% have venographically confirmed total DVT.14
Recommended pharmacologic options for thromboprophylaxis after THA or TKA include vitamin K antagonists (e.g., warfarin), low-molecular-weight heparins (e.g., enoxaparin, dalteparin), and an indirect Factor Xa inhibitor (fondaparinux). Limitations of warfarin include the requirement for regular coagulation monitoring, the potential for food and drug interactions, and a delayed onset of action.14,46 Parenteral anticoagulants, such as enoxaparin and fondaparinux, may be less convenient than those administered orally. A risk of heparin-induced thrombocytopenia is associated with low-molecular-weight heparins.15
To overcome these limitations, new oral anticoagulants that do not require regular coagulation monitoring, are unlikely to interact with food and drugs, and have a fast onset of action are being researched. The trend toward short hospital stays of less than 4 days14 and the finding that most symptomatic events occur at a mean of 10 days after TKA and 22 days after THA (Fig. 142-1) mean that thromboprophylaxis is as important after hospital discharge as before.45 Because patients may need to self-administer thromboprophylaxis at home, the ideal anticoagulant would have to be easy to administer. On this point, oral anticoagulants have a clear advantage over parenteral anticoagulants.
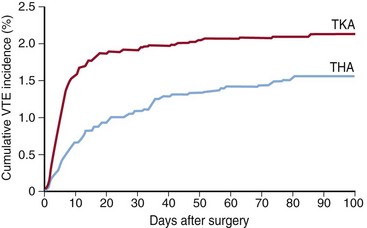
Figure 142-1 Cumulative incidence of venous thromboembolism after total hip or knee arthroplasty.45 THA, Total hip arthroplasty; TKA, total knee arthroplasty.
(Reproduced with permission and copyright © of the British Editorial Society of Bone and Joint Surgery [Warwick D, Friedman RJ, Agnelli G, et al: J Bone Joint Surg Br 89:799–807, 2007].)
Several new oral anticoagulants in development have shown promising benefit–risk profiles in clinical trials.31,32,44,47 Great interest has centered around the use of oral, direct Factor Xa inhibitors such as apixaban and rivaroxaban and a direct thrombin inhibitor—dabigatran etexilate—for thromboprophylaxis in an orthopedic setting. This chapter introduces these new oral agents and discusses important findings from clinical trials conducted in patients undergoing THA and TKA.
Direct Factor Xa Inhibitors
Mode of Action
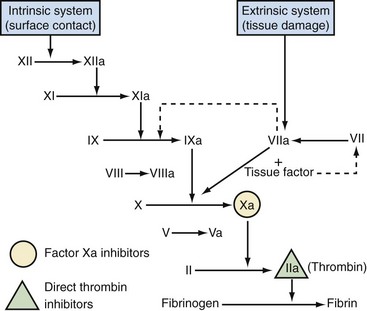
The enzyme Factor X is an attractive target for inhibition, as it occupies a critical junction between the intrinsic and extrinsic coagulation cascade pathways, and is essential for the conversion of prothrombin to thrombin, which leads to thrombus formation33,34 (Fig. 142-2). In response to vascular injury, Factor X is activated to Factor Xa by the contact (intrinsic pathway) or by the tissue factor/Factor VIIa (extrinsic pathway).33 Factor Xa combines with its cofactor Factor Va on phospholipid membranes to form the prothrombinase complex.33 This complex converts prothrombin to thrombin, which leads to the amplified generation of thrombin.
Indirect Factor Xa inhibitors, such as fondaparinux, have demonstrated that Factor Xa is an effective target for anticoagulation. Direct Factor Xa inhibitors have some advantages over indirect Factor Xa inhibitors: unlike indirect Factor Xa inhibitors (which catalyze Factor Xa inhibition by antithrombin), direct Factor Xa inhibitors bind directly to Factor Xa, thus preventing the subsequent reactions that lead to thrombin generation.46 In addition, they inhibit both free and platelet-bound Factor Xa, as well as Factor Xa bound to the prothrombinase complex.46 Some direct Factor Xa inhibitors in development (e.g., DX-9065a) are administered parenterally, whereas others (e.g., apixaban, rivaroxaban) are orally active. An oral route of administration provides added convenience and could encourage patient compliance (particularly in the outpatient setting). Apixaban and rivaroxaban are oral, direct Factor Xa inhibitors that have been studied as anticoagulants after THA and TKA, and phase III studies have been completed for both agents. Currently, rivaroxaban has been approved in Canada and several other countries for the prevention of VTE in adult patients undergoing elective hip or knee arthroplasty.2
Apixaban
Pharmacokinetics and Pharmacodynamics
Apixaban was designed as a follow-up compound to razaxaban, an oral, direct Factor Xa inhibitor that was discontinued because of its suboptimal pharmacologic profile.5 Apixaban is orally bioavailable and is absorbed rapidly. It has a mean terminal half-life (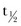 ) of approximately 13 hours.37 Approximately 50% of administered apixaban is absorbed. In human plasma, apixaban concentration dependently prolongs the standard clotting assays activated by partial thromboplastin time (aPTT), prothrombin time (PT), and the HepTest (American Diagnostica, Stamford, Conn).11
) of approximately 13 hours.37 Approximately 50% of administered apixaban is absorbed. In human plasma, apixaban concentration dependently prolongs the standard clotting assays activated by partial thromboplastin time (aPTT), prothrombin time (PT), and the HepTest (American Diagnostica, Stamford, Conn).11
Phase III Studies
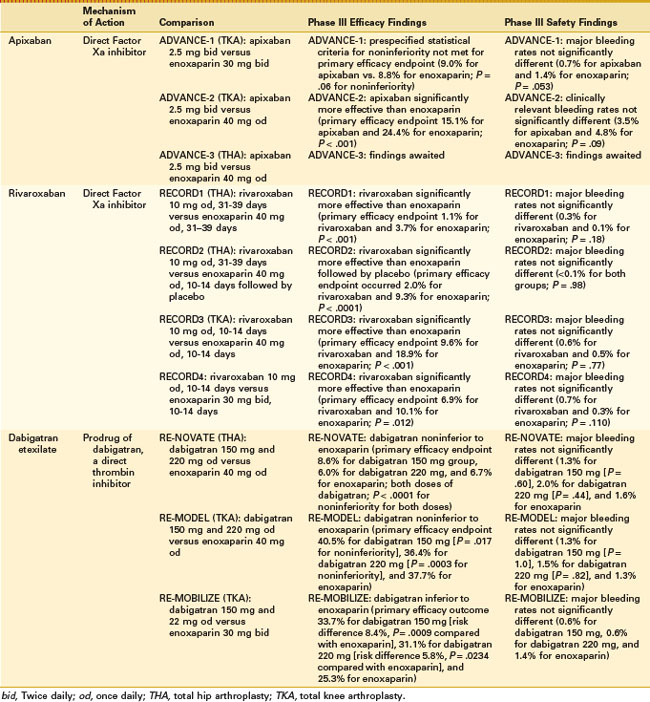
Two phase III, randomized, double-blind studies compared apixaban with enoxaparin regimens for thromboprophylaxis after TKA (Table 142-1). In the ADVANCE-1 study (n = 3195), apixaban (2.5 mg twice daily, started 12 to 24 hours after surgery) was compared with the North American enoxaparin regimen (30 mg every 12 hours, started 12 to 24 hours after surgery).32 The primary efficacy endpoint was the composite of DVT (symptomatic/asymptomatic), nonfatal PE, and death by any cause during the drug administration period. In patients who received apixaban, the rate of the primary efficacy endpoint was numerically similar to that in patients who received enoxaparin (9.0% vs. 8.8%; absolute risk difference, −0.1%; P = .06 for noninferiority), but the prespecified statistical criteria for noninferiority compared with enoxaparin were not met. Rates of symptomatic VTE and VTE-related death were 1.2% in the apixaban group and 0.8% in the enoxaparin group. Rates of major bleeding were 0.7% in the apixaban group versus 1.4% in the enoxaparin group (P = .053). Rates of major plus clinically relevant nonmajor bleeding were 2.9% in the apixaban group and 4.3% in the enoxaparin group (P = .03). The bleeding definitions were different for apixaban, rivaroxaban, and dabigatran; therefore no direct comparison can be made between the bleeding data for these drugs. The incidence of liver enzyme elevations (alanine aminotransferase and aspartate aminotransferase >3 times the upper limit of the normal range) was low in both groups, and the incidence of adverse events was similar between groups.
The ADVANCE-2 study (n = 3057) compared the same apixaban regimen with the European enoxaparin regimen (40 mg once daily).31 The primary efficacy endpoint, which had the same definition as in ADVANCE-1, occurred in 15.1% of patients receiving apixaban versus 24.4% of those receiving enoxaparin (absolute risk difference, −9.3%; one-sided P < .001). Clinically relevant bleeding (major and nonmajor) occurred in 3.5% and 4.8% of patients given apixaban and enoxaparin, respectively (P = .09). Rates of liver enzyme elevations (defined as in ADVANCE-1) and adverse events were low and similar between groups.
Rivaroxaban
Pharmacokinetics and Pharmacodynamics
Rivaroxaban is an oral, direct Factor Xa inhibitor,38 which is rapidly absorbed. It has a mean terminal 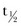 of 7 to 11 hours.2 Of the administered dose, approximately two thirds undergoes metabolic degradation, with half then being eliminated renally and the other half eliminated by the fecal route. The final one third of the administered dose undergoes direct renal excretion as unchanged active substance in the urine, mainly via active renal secretion.2 Rivaroxaban was found to be well tolerated and to have predictable, dose-proportional pharmacokinetics and pharmacodynamics across a wide range of doses (5 to 80 mg single doses) in healthy individuals23,24; this suggests that rivaroxaban does not require regular coagulation monitoring.2 Based on phase II data, rivaroxaban plasma concentrations increase dose dependently. Factor Xa activity and PT correlate with rivaroxaban plasma concentrations.35,36 Weight, gender, age, and renal function do not substantially affect the pharmacokinetics or pharmacodynamics of rivaroxaban.19,25 At the 10 mg once-daily dose used in phase III clinical studies for thromboprophylaxis after THA and TKA, food does not significantly affect rivaroxaban pharmacokinetics.2 No clinically relevant interactions were observed between rivaroxaban and several frequently used concomitant medications,18,20–22,26–28 but coadministration of strong inhibitors of both CYP3A4 and P-glycoprotein (e.g., ketoconazole, itraconazole, voriconazole, posaconazole, ritonavir) may increase plasma concentrations of rivaroxaban, and strong CYP3A4 inducers (e.g., rifampicin, phenytoin, carbamazepine, phenobarbital, St John’s Wort) may reduce rivaroxaban plasma concentrations when coadministered.2
of 7 to 11 hours.2 Of the administered dose, approximately two thirds undergoes metabolic degradation, with half then being eliminated renally and the other half eliminated by the fecal route. The final one third of the administered dose undergoes direct renal excretion as unchanged active substance in the urine, mainly via active renal secretion.2 Rivaroxaban was found to be well tolerated and to have predictable, dose-proportional pharmacokinetics and pharmacodynamics across a wide range of doses (5 to 80 mg single doses) in healthy individuals23,24; this suggests that rivaroxaban does not require regular coagulation monitoring.2 Based on phase II data, rivaroxaban plasma concentrations increase dose dependently. Factor Xa activity and PT correlate with rivaroxaban plasma concentrations.35,36 Weight, gender, age, and renal function do not substantially affect the pharmacokinetics or pharmacodynamics of rivaroxaban.19,25 At the 10 mg once-daily dose used in phase III clinical studies for thromboprophylaxis after THA and TKA, food does not significantly affect rivaroxaban pharmacokinetics.2 No clinically relevant interactions were observed between rivaroxaban and several frequently used concomitant medications,18,20–22,26–28 but coadministration of strong inhibitors of both CYP3A4 and P-glycoprotein (e.g., ketoconazole, itraconazole, voriconazole, posaconazole, ritonavir) may increase plasma concentrations of rivaroxaban, and strong CYP3A4 inducers (e.g., rifampicin, phenytoin, carbamazepine, phenobarbital, St John’s Wort) may reduce rivaroxaban plasma concentrations when coadministered.2
Phase III Studies
The REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism (RECORD) program comprised four phase III trials, which included more than 12,700 randomized patients with no age or weight limit7,16,30,43 (see Table 142-1). All four studies were prospective randomized, double-blind, double-dummy, controlled trials. RECORD1 and RECORD2 were conducted in patients undergoing THA, and RECORD3 and RECORD4 in patients undergoing TKA. The primary efficacy endpoint in all four trials was the composite of symptomatic and asymptomatic DVT (detected by venography), nonfatal PE, and all-cause mortality. Rivaroxaban was started 6 to 8 hours after wound closure in each trial. In RECORD1, 2, and 3, enoxaparin was started 12 hours before surgery and was restarted 6 to 8 hours after wound closure; in RECORD4, enoxaparin was started 12 to 24 hours after wound closure.
RECORD2 (n = 2509) compared extended prophylaxis with rivaroxaban 10 mg once daily for 31 to 39 days with short-duration enoxaparin prophylaxis (40 mg once daily) for 10 to 14 days with placebo tablets for 31 to 39 days.16,17 The primary efficacy endpoint occurred in 2.0% of the rivaroxaban group versus 9.3% of the enoxaparin group (absolute risk reduction, 7.3%; P < .0001). Major bleeding occurred in less than 0.1% of patients in both groups (P = .98). Symptomatic VTE occurred in 0.2% of the rivaroxaban group and 1.2% of the enoxaparin group (P = .004). No patients experienced bleeding leading to reoperation. Rates of postoperative wound infections were 0.7% in the rivaroxaban group and 0.5% in the enoxaparin group; rates of hemorrhagic wound complications were 1.6% and 1.7%, respectively.
RECORD3 (n = 2531) compared rivaroxaban 10 mg once daily with enoxaparin (40 mg once daily) for 10 to 14 days.30 In the rivaroxaban group, the primary efficacy endpoint occurred in 9.6% of patients, and in the enoxaparin group, it occurred in 18.9% of patients (absolute risk reduction, 9.2%; P < .001). Major bleeding rates were 0.6% for rivaroxaban and 0.5% for enoxaparin (P = .77). Rates of symptomatic VTE were 0.7% in the rivaroxaban group and 2.0% in the enoxaparin group (P = .005). The incidence of bleeding leading to reoperation was 0.4% in the rivaroxaban group and 0.3% in the enoxaparin group. Postoperative wound infections occurred in 0.6% of the rivaroxaban group and 0.9% of the enoxaparin group, and hemorrhagic wound complications occurred in 2.0% and 1.9%, respectively.
RECORD4 (n = 3148) compared rivaroxaban 10 mg once daily with the North American enoxaparin regimen (30 mg every 12 hours) for 10 to 14 days.43 The primary efficacy endpoint occurred in 6.9% of the rivaroxaban group versus 10.1% of the enoxaparin group (absolute risk reduction, −3.19%; P = .012). Rates of major bleeding were 0.7% for rivaroxaban and 0.3% for enoxaparin (P = .110). Symptomatic VTE occurred in 0.7% of the rivaroxaban group compared with 1.2% of the enoxaparin group (P = .187). Bleeding leading to reoperation occurred in 0.3% of the rivaroxaban group and 0.1% of the enoxaparin group. Rates of postoperative wound infections were 0.3% versus 0.2%, respectively, and hemorrhagic wound complications were 1.4% and 1.5%, respectively.
The RECORD studies showed that rivaroxaban (10 mg once daily) was significantly more effective than the European and North American enoxaparin regimens at preventing the composite of DVT, nonfatal PE, and all-cause mortality, without significantly increasing the risk of major bleeding. Also, rates of the combination of major and clinically relevant nonmajor bleeding were low for rivaroxaban and enoxaparin (P = .206 [data on file], P = .394 [data on file], P = .43930 [data on file], and P = .17943 for RECORD1, RECORD2, RECORD3, and RECORD4, respectively). Incidences of bleeding leading to reoperation, postoperative wound infections, liver enzyme elevations, and cardiovascular adverse events were similar between groups. A trend for reduction in the incidence of symptomatic VTE was seen with rivaroxaban, and this reached statistical significance in the RECORD2 and RECORD3 studies.
In a pooled analysis of the three trials that compared rivaroxaban with enoxaparin 40 mg once daily (RECORD1, 2, and 3),10 the prespecified primary efficacy outcome (the composite of symptomatic VTE [DVT or PE] and all-cause death at 2 weeks, which included the enoxaparin-controlled period of all three studies to allow for an unbiased comparison with enoxaparin) was 0.4% and 0.8% for rivaroxaban and enoxaparin, respectively (P = .005). Rates were 0.5% and 1.3%, respectively, at the end of the planned medication period (up to day 42 in RECORD1 and RECORD2, including the enoxaparin placebo period in RECORD2, and up to day 17 in RECORD3) (P < .001). On-treatment major bleeding occurred in 0.2% of patients for both drugs at 2 weeks (P = .662) and in 0.3% of those assigned rivaroxaban and 0.2% of those assigned enoxaparin at the end of the planned medication period (P = .305). Clinically relevant nonmajor bleeding rates were 2.6% for rivaroxaban and 2.3% for enoxaparin at 2 weeks, and 3.0% and 2.5%, respectively, at the end of the planned medication period (P values not reported).
In a pooled analysis of data from RECORD1 through 4,44 rivaroxaban reduced the incidence of the composite of symptomatic VTE and death compared with the enoxaparin regimens, with no differences in rates of major bleeding. In a post hoc analysis, rates of the composite of PE and death were lower for rivaroxaban than for enoxaparin during the planned treatment period and follow-up.13 Subgroup analyses indicated that rivaroxaban was superior to enoxaparin irrespective of age, weight, gender, or renal function (for creatinine clearance categories above 30 mL/min).1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree