CHAPTER 81 Acute Treatment of Patients with Spinal Cord Injury
Since the implementation of emergency medical systems and model spinal cord injury care systems in the early 1970s, the mortality rate from spinal cord injury during the initial period of hospitalization has decreased fivefold.1 This reduction primarily reflects improvements in the initial management of these injuries, which still account for about 10,000 to 12,000 new cases each year.2–4 The improved care at time of injury and thereafter has increased life expectancies. Consequently, the current prevalence of about 250,000 people with spinal cord injury in the United States will continue to grow.5,6 The cost to society, in both health care expenses and living expenses, is almost $8 billion per year. Lifetime expenses per patient range from $435,000 to more than $2.6 million.6,7 That the average age of spinal cord–injured patients is about 30 years further increases lifetime expenses.3,8
Since 2005 the most frequent cause of spinal cord injury in the United States has been motor vehicle crashes (42%), followed by falls (27.1%). Recently, injuries from falls have increased the most. The proportion of injuries related to violence (15.3%) has decreased from its peak in 1999 (24.8%), and the proportion of sports-related injuries has also diminished (7.4%).4 About 50% of victims have a complete neurologic deficit below the level of injury and require significant assistance with activities of daily living.8 Prevention is the single best remedy for spinal cord injuries. Nonetheless, advances in the acute management of these injuries have had the greatest effect on improving long-term outcomes.1
Prehospital Management
Cervical Spine Immobilization
Evidence from the past 30 years has shown that patients should be placed in rigid external cervical spine immobilization when extricated at the scene.1,9,10 The rationale for immobilization is predicated on the theory that neurologic function can be compromised by pathologic motion of injured vertebrae. Although logical, no class I evidence supports this supposition, and controversy surrounds the effectiveness of prophylactic spinal immobilization.11,12
Several estimates indicate that 3% to 26% of spinal cord injuries occur after the initial injury, presumably caused by abnormal movement of the spinal column and subsequent damage to neural structures.9,13–15 If so, a large number of cervical spinal cord injuries might be prevented by earlier immobilization of the cervical spine. Supporting this assertion is the dramatic decrease in the percentage of complete injuries (as opposed to incomplete) in the past 30 years.16,17 Prehospital immobilization has not been the only major change during this period, but it is thought to have been a major contributor to this decrease.
The large number of traumatically injured patients in the United States each year raises the issue of which patients need spinal immobilization. The routine use of immobilization to prevent unnecessary injuries makes sense. However, only a small percentage of immobilized patients actually have a significant spinal injury. The decision to immobilize a patient is usually based on the mechanism of injury and the patient’s clinical status. The following clinical criteria for a potential spinal injury are most predictive of the need for immobilization18: an altered mental status; focal neurologic deficits; evidence of intoxication, spinal pain, or tenderness; and suspected extremity fracture. Even when these criteria are strictly applied, up to 5% of spinal injuries may still be missed.19
Although techniques of spinal immobilization may vary, guidelines from the American College of Surgeons recommend that a hard backboard, rigid cervical collar, and lateral support device be used, with tape or straps to secure the patient, collar, and lateral support devices to the backboard.20 The general goal for adequate immobilization is the “neutral position,” which is the normal anatomic position of the head and torso when standing and looking forward.21 Although these recommendations are straightforward, there is some controversy about the appropriate position to attain optimal spinal stability. The neutral position is usually attained by providing about 12 degrees of cervical spine extension on a lateral radiograph. However, this position may not offer the best alignment. Elevating the occiput 2 cm to create a slight flexion provides more room for the spinal cord at C5-6, which is the spinal level most commonly injured.22 However, the large variability in body habitus precludes rigid guidelines for the use of occipital padding.
Spinal immobilization is not a completely benign process, and its limitations must be recognized. A large portion of patients experience moderate to occasionally severe pain after relatively short periods (30 minutes) of immobilization on a rigid backboard.23 Relieving this discomfort with a small amount of padding has no effect on the level of immobilization and may minimize patient movements related to pain.24 Another concern with immobilization is the increase in intracranial pressure (ICP) related to the use of rigid cervical collars.25,26 Although such increases are relatively small (4.5 mm Hg and 24.7 cm H2O), they may be clinically significant in patients with severe head injuries whose ICP is already elevated. Furthermore, immobilization on a rigid, nonpadded backboard has been associated with the development of pressure sores. Not surprisingly, this relationship is related to the length of time spent on the backboard: the presence of ulcers increases after 2 or more hours on the board.27
Transportation of Spinal Cord–Injured Patients
Many advances in the transportation of spinal cord–injured patients reflect protocols implemented after the Vietnam War. The most significant advance was rapid transportation to a specialized center for definitive care. In the United States and Canada this system has grown into the trauma level system at a regional basis. Several studies have shown significant benefits associated with both rapid transport times to definitive medical centers and improved neurologic function at admission.28–30 Although patients fare better when transported quickly to definitive centers, unstable patients may still be better served by transport to a regional center until stabilized and then later transported to the appropriate tertiary center.20 Because there are no prospective, randomized trials (and unlikely to be any) on this issue, the treatment of each case must be individualized.
The type of transportation available usually depends on the region, but most areas in the United States are served by both air and ground transportation. In rural areas, air transportation providing rapid arrival at tertiary care centers can dramatically decrease mortality rates in trauma patients.31 In a retrospective analysis at a single institution, there was no difference in the neurologic outcomes of patients transported by ground or air.32 Thus both air and ground transportation appear to be equally safe, and the decision to use one or the other depends on availability and speed.
Various transfer techniques from the scene of the accident or between vehicles and different support equipment (e.g., backboards, gurneys, or beds for magnetic resonance imaging [MRI] or computed tomography [CT]) are available. Some examples include the log roll, the Haines maneuver, and the multihand (fireman’s) lift.20,33,34 All are equally effective for moving patients from the scene of an accident to a backboard. The choice should depend on the individual circumstances.
Clinical Assessment
Even when the suggestion of a spinal cord injury is high, at the initial clinical assessment the examiner must consider the basics involved with evaluation of traumatically injured patients. The initial evaluation consists of the primary and secondary surveys from the Advanced Trauma Life Support (ATLS) guidelines.20 The primary survey is usually initiated at the accident scene and is focused on the basics of life support: airway, breathing, and circulation. Once these issues have been addressed, the secondary survey is used to evaluate patients for significant injuries from head to toe.
An adequate airway and ventilation are the most important factors in the initial survey of traumatically injured patients. An injured spinal cord is susceptible to further injury from hypoxemia. Even if patients are alert and breathing adequately, supplemental oxygen is recommended if a spinal cord injury is suspected. In obtunded patients with a suspected spinal cord injury, early placement of an endotracheal or nasotracheal tube is critical to limit secondary injury from hypoxemia. A dedicated airway can be placed safely in patients with a cervical spine injury, although neck extension should be minimized during its placement.35
Treatment of hypotension is part of the resuscitation phase of the ATLS protocol, which parallels the primary and secondary surveys. Immediate aggressive treatment of hypotension is important to maintain adequate perfusion to the injured spinal cord to reduce further secondary injury from tissue ischemia.36 However, patients in neurogenic shock should not be overloaded with fluid. This problem is most common in elderly patients who have less cardiovascular capacity for high intravascular volumes than younger individuals. Once patients reach a dedicated trauma center, pressors and intravascular fluid monitors such as a Swan-Ganz catheter can be used to control blood pressure within a narrow range.
Neurologic Assessment
After the initial evaluation of traumatically injured patients, it is crucial to determine the presence of a spinal cord or column injury. A rapid examination in the secondary survey usually unmasks pronounced neurologic deficits, but a more detailed neurologic examination may be necessary to uncover subtle deficits. Once patients’ respiratory and cardiovascular systems are stabilized, a full neurologic examination can be performed. The examination must incorporate established classification scales for spinal cord injury. Outside specific clinical trials, the most widely used spinal cord injury scales are the Frankel and American Spinal Injury Association (ASIA) scales (Table 81–1). In fact, recent guidelines recommend use of the ASIA scale, although strictly as an option.37
A detailed neurologic assessment not only helps classify an injury but also guides further radiographic assessment and treatment. The neurologic examination will determine whether an injury is complete or incomplete. With the improvements in prehospital emergency systems during the past 30 years, the ratio of incomplete-to-complete injuries has increased considerably.16,17 An incomplete injury is a predictor of a better outcome than a complete injury. Therefore urgent treatment is assumed to be necessary, although no randomized trials have verified this assumption.38
Spinal Cord Injury Syndromes
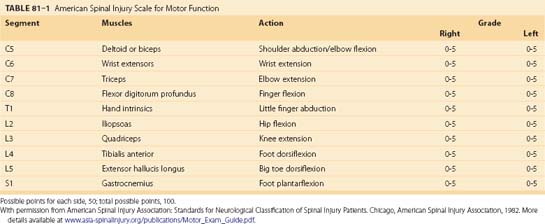
Several types of incomplete spinal cord injuries are based on the functional anatomy of the spinal cord in relation to the mechanism of injury. A common type of incomplete spinal cord injury is the central cord syndrome, which is identified by more motor and sensory deficits involving the upper extremities than the lower extremities.39 Initially, a burning dysesthesia of the upper extremities often accompanies the other deficits and gradually resolves within a few days. This injury is unique to the cervical spinal cord with the pattern of injury related to edema of the central gray matter at the cervical level. This edematous reaction is caused by crushing or stretching the spinal cord in patients with significant stenosis, either congenital or degenerative. Hyperextension combined with stenosis is the most common mechanism underlying this injury. A similar injury occurs in children while their interlaminar and intervertebral ligaments are still lax enough to allow extreme hyperextension. An acute traumatic cervical disc herniation can also cause the same type of injury.
Central cord syndrome causes multilevel dysfunction in motor and sensory neurons at the cervical level while relatively sparing the axons communicating with lower extremity neurons. This pattern is further compounded by the medial location of axons innervating the cervical neurons compared with the lateral location of lumbar neurons. The central portion of the spinal cord may be susceptible because the area is a vascular watershed.40 In the most severe cases, the edema leads to severe ischemia, capillary destruction, and hematomyelia. The most useful imaging study for these patients is a T2-weighted MRI sequence, which can visualize edema and hemorrhage, if present (Fig. 81–1). Acute decompression is recommended for patients with an acutely herniated disc or traumatic fracture.41 In patients with cervical stenosis or spondylosis, we recommend waiting until the initial edema recedes or until the neurologic impairment has plateaued. Depending on the pathology, anterior or posterior decompression can then be performed.
A rare type of spinal cord injury is the Brown-Séquard syndrome, or hemicord syndrome. It is usually caused by penetrating injuries but may be associated with lateral bony fractures or acutely herniated disc fragments. The hallmark of the syndrome is ipsilateral motor deficits and dorsal column dysfunction and contralateral spinothalamic dysfunction.42 It is rarely seen in isolation, which sometimes makes it difficult to diagnose definitively.
Radiographic Assessment
One of the earliest dilemmas in evaluating traumatically injured patients is how far the radiographic analysis should proceed to rule out spinal injury. In the current medicolegal environment, this decision can be daunting, given the cost of some of the studies involved. Patients with no neurologic symptoms or signs, no spinal pain or tenderness, no distracting injuries, and no impairment by drugs or ethanol should require no radiographic assessment.43
The most compelling support for this guideline is from the National Emergency X-radiography Utilization Study Group (NEXUS) in 2000.44 The study called for three-view radiography, CT, and MRI as needed in each patient to rule out spinal injuries. Of 34,069 patients enrolled, 4309 met the asymptomatic criteria. Only two patients were found to have a clinically significant spinal column injury, and none was found to have significant neurologic abnormalities. On the basis of the negative predictive value of 99.9% and the positive predictive value of 1.9%, the current standard is to clear asymptomatic patients of cervical spine injuries on the basis of the clinical examination alone if the previously mentioned criteria are met. This guideline, however, is valid only for cervical spine clearance and must be extrapolated carefully to patients with potential thoracic and lumbar injuries. The presence of thoracolumbar fractures, however, is always associated with pain in awake patients because significant force is necessary to produce fractures at these levels.
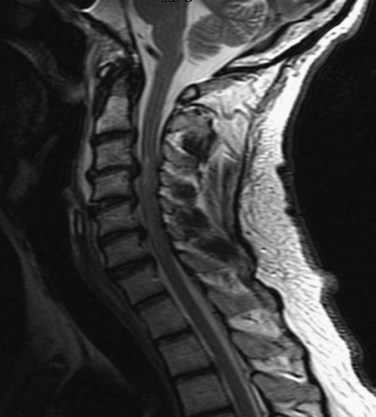
In all other symptomatic patients, an initial radiographic analysis should be performed with lateral, anteroposterior, and open-mouth views of the cervical spine. Thoracic and lumbar two-view radiographs should be obtained in patients with pain in these regions or who have a significant mechanism of injury. In patients with blunt trauma, these screening studies are critical because the rate of significant cervical, thoracic, or lumbar spine injury ranges between 2% and 6%.45,46 When properly performed, simple screening cervical radiography has a negative predictive value between 93% and 98%.47–49 The most common cause of a missed cervical spine injury is the failure to visualize the area of injury adequately. Therefore the spine must be imaged from the occiput to the first thoracic vertebrae (Fig. 81–2).
Over the past 20 years, the use of CT to evaluate for spinal injuries after trauma has increased significantly. Currently, CT is the modality most often used to assess areas not adequately visualized with radiography. This combination leads to an almost 100% sensitivity in detecting significant spinal injuries in symptomatic patients.50 The increased cost of CT compared with radiography is the main issue involved with its use. CT, however, is cost effective when used as an adjunct to radiography.51 We routinely obtain cervical CT scans in symptomatic trauma patients, and almost all comatose patients undergo CT of the cervical, thoracic, and lumbar spine on admission.
Rapid advances in MRI technology and the recent increased availability of scanners have led to the use of this modality for the evaluation of spinal injuries in trauma patients. Although the ability of MRI to image bony structures is limited, it is sensitive to abnormalities in soft tissue, especially ligamentous structures, intervertebral discs, and the spinal cord itself. A study by the Eastern Association for the Surgery of Trauma found a high rate of detection of cervical abnormalities in trauma patients using MRI.52 Because MRI is extremely sensitive to any soft tissue injury, the rate of false-positive scans for significant spinal injury in trauma patients is thought to be elevated.53 However, MRI can help determine the absence of spinal injury because the rate of false-negative findings is low when used acutely.54 Consequently, we believe that MRI should be used in the presence of a neurologic deficit, if time permits, or to rule out any type of spinal injury when other studies are inconclusive or unavailable.
Trauma patients must also be screened for the presence of instability. In the thoracic and lumbar spine, the presence of instability is usually related to significant bony trauma. Instability in the cervical spine can be subtle such as a hairline fracture of a facet or ligamentous injury that may be undetected on radiography or even CT. Symptomatic or obtunded patients should always be evaluated for cervical instability. The best test is an adequate series of flexion-extension lateral radiographs. Two recent studies analyzed the efficacy of flexion-extension views in trauma patients.55,56 Both showed a low rate of false-negative findings (99% and 100% negative predictive value, respectively), but both were limited by inadequate studies in up to 30% of the examinations. When the incidence of inadequate studies is considered, the sensitivity of this modality remains in question. If dynamic radiographs are inadequate or inconclusive, MRI can be used to rule out instability but not necessarily to diagnose it because of the high rate of false-positive findings.54 For comatose patients, the options to determine instability are MRI or passive dynamic radiography with fluoroscopy. Passive dynamic radiographs can be a viable first option, as shown by the relatively high number of obtunded patients with unstable cervical spines discovered by this method.57
Clinical Management
The Edwin Smith papyrus, the world’s oldest surgical document, states that an injury of the spinal cord that renders the patient “unconscious of his two legs and his two arms” is “an ailment not to be treated.”58 Although outcomes from spinal cord injuries are still usually poor, our understanding of these injuries and potential treatments has advanced considerably in the 5000 years since this document was written.
Hypoxemia in spinal cord injury can be caused by many factors. The two most common causes are decreased respiratory drive due to unconsciousness and decreased mechanics of ventilation due to a cervical spinal cord injury. In each case, early intubation and aggressive oxygenation should be implemented. Some authors recommend oxygenation to a PaO2 of more than 100 mm Hg, at least for an initial period.10 Patients with a complete injury above C5 should seriously be considered for prophylactic intubation because of the high rate of ventilatory decompensation in these patients.59 In patients with adequate initial ventilation and an injury at a lower level of the spinal cord, noninvasive measures can be used to ensure adequate oxygenation. These measures include monitoring oxygen saturation and periodic PaO2 levels in an intensive care setting. The authors also recommend other measures such as early nasogastric decompression, head-of-bed elevation if possible, and early start of a bowel regimen to assist with respiratory effort by decreasing the work of breathing. If ventilatory decompensation begins, prophylactic intubation should be undertaken to prevent hypoxemia and its additive effect on the secondary injury cascade.60
Hypotension can be related to the spinal cord injury itself or to hypovolemia from another traumatic injury. When sympathetic outflow is disrupted in the cervical or upper thoracic spinal cord, peripheral vascular resistance decreases profoundly, leading to hypotension. This is the primary mechanism underlying neurogenic shock and can usually be differentiated from hypovolemic shock by the relative lack of a tachycardic response. These patients must be treated aggressively to avoid prolonged hypotension and accentuation of the secondary injury cascade. The effect of hypotension is compounded by the dysfunctional hemodynamic autoregulation found in the spinal cord parenchyma immediately after a traumatic injury.61 Animal studies have shown that maintaining adequate systemic blood pressure maintains spinal cord perfusion pressure in the physiologic range and limits the amount of ischemic damage to the site of injury.62
To address the hemodynamic alterations that follow spinal cord injury, several prospective studies have evaluated the role of hemodynamic stabilization and its effect on long-term outcomes after spinal cord injury.30,36,63 One study used invasive hemodynamic monitoring with Swan-Ganz catheters and aggressively treated hypotension with fluids, volume expanders, and vasopressors to maintain mean arterial pressure above 85 mm Hg for the first 7 days after injury.36 This aggressive management led to better-than-expected neurologic outcomes including 30% of patients with a complete cervical spinal cord injury regaining the ability to walk. However, none of these studies included control groups, owing to the obvious ethical problems. Based on the extensive experimental evidence and prospective clinical data, systemic blood pressure should be maintained above a mean of 85 to 90 mm Hg immediately after injury and for at least 1 week thereafter.