Susan Craig Scott
W. Norman Scott
Wound Complications
INTRODUCTION
Rapid and uncomplicated wound healing is critical for successful results after both primary and revision total knee replacement. Failure to achieve early wound healing increases the risks of infection and prosthesis failure and may result in additional surgical procedures. Furthermore, problems with wound healing may interfere with rehabilitation and hospital dismissal. Consequently, wound healing problems may not only lead to increased costs but also increased morbidity and diminished long-term function. Certainly, early identification and treatment is required whenever suboptimal wound healing occurs. However, it is not sufficient to wait for problems to develop postoperatively; rather, strategies must be employed that identify patients at risk for wound healing problems and allow prophylactic measures to be taken not only preoperatively but also intraoperatively and postoperatively. Preoperative evaluation should include an assessment of both the systemic conditions and the local environment of the knee that are specific to the patient and that may impede healing. As a result of this evaluation, any factors that can be optimized prior to surgery should be addressed. In addition, any required changes in intraoperative or postoperative management should be planned prior to surgery in order to minimize complications.
ANATOMY
In order to better understand how systemic and local factors can influence wound healing, it is important to consider the anatomy of the knee, especially the vascular supply to the skin and deep tissues. The deep tissues of the anterior knee, including retinaculum, extensor mechanism, and capsule, receive arterial flow via terminal branches of the anterior anastomosis (Fig. 50-1). This anastomosis is formed by the four inferior and superior genicular arteries from the popliteal artery, branches of the descending genicular artery, descending branch of the lateral circumflex femoral artery, and recurrent branches of the anterior tibial artery.1,2 The anterior anastomosis thus connects the femoral artery at the origin of its profundus branch with the popliteal and anterior tibial arteries. The arterial supply to the overlying skin is from three sources, including direct cutaneous, musculocutaneous, and septocutaneous (intermuscular) vessels.3–5 These vessels include random perforating and axial-type distribution. The perforating vessels include terminal branches from the anterior anastomosis, as well as additional musculocutaneous terminal branches from the arterial supply to the rectus femoris and vastus muscles. Once they perforate through the deep fascia, these vessels course parallel to the skin surface for a considerable distance in the loose areolar layer that separates the deep fascia from the subcutaneous fat and form an interconnecting fascial plexus (Fig. 50-2).4,6 Branches from this fascial plexus traverse the subcutaneous tissue and anastomose with other branches creating a subdermal plexus in the skin.6,7 As the skin relies on the distribution from the fascial plexus that is just superficial to the deep fascia, undermining of the skin and creation of elevated skin and subcutaneous flaps should be minimized. Indeed, the true surgical plane of the anterior knee appears to be beneath the deep fascia.7 Furthermore, although the skin receives arterial inflow that is provided by both medial and lateral contributions to the anterior anastomosis, it is clear that the primary vascular supply originates from the medial side.2,4 In particular, the saphenous artery, which arises in a common trunk with the descending genicular vessel from the superficial femoral artery, provides a major contribution to the fascial plexus.2,4 Indeed, the predictability of this axial-type contribution to the arterial supply of the skin and subcutaneous tissue over the anteromedial knee and lower leg has allowed the development of fasciocutaneous, adiopofascial, and free flaps based upon the saphenous artery.4,8 It is also clear that due to this medially biased arterial inflow, large lateral flaps should be avoided.2,9,10
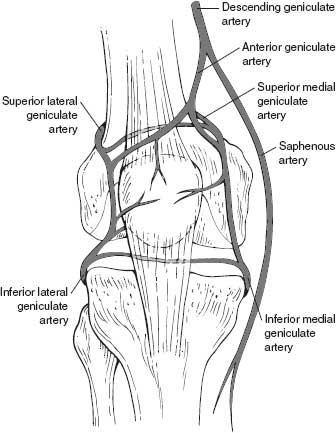
FIGURE 50-1. The anterior arterial anastomosis.
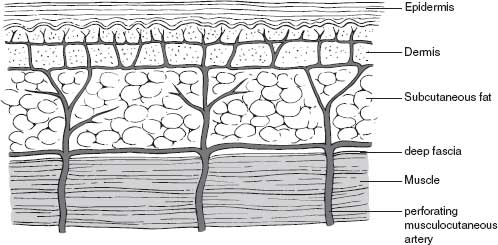
FIGURE 50-2. Arterial circulation of the skin about the knee.
PREOPERATIVE EVALUATION
Numerous reports have documented the effects of both systemic conditions and local factors on wound healing about the knee. Preoperative evaluation and optimization of these factors may help prevent suboptimal healing.
Systemic Factors The importance of adequate nutrition for appropriate wound healing and recovery after surgery has been well described.11–14 A total lymphocyte count <1,500 per mm3, serum albumin <3.4 g/dL, and recent weight loss exceeding 10 lb have been defined as indicators of poor nutritional levels associated with wound healing problems.12,13,15 A higher rate of wound healing complications has also been noted in patients with underlying malignant disease, likely due to the overall debilitated state and malnutrition seen in this group of patients.16 The association between obesity and wound healing is less well defined, although a significant increase in wound problems has been noted.11,17 Wong et al.17 reported a linear relationship between increasing weight and wound problems following total knee replacement. Wilson et al.18 also reported a higher incidence of infection in obese patients, but the increase was not statistically significant. In a similar report, Stern and Insall19 did not identify any increase in wound-related complications. The increased rate of wound complications may relate to technical factors such as vigorous retraction required for surgical exposure resulting in disruption of the fascial plexus rather than systemic effects such as subtle immune compromise.
Increased wound complications and infection have also been noted in patients with rheumatoid arthritis.17,18,20,21 It is difficult to determine whether these effects are independent of the corticosteroids used in this population.18,20 Poor wound healing in patients with rheumatoid arthritis may be related to the atrophic skin, decreased albumin, and vasculitis encountered in these patients.20 Higher infection rates may be due to inhibited neutrophil function.18 Diabetes mellitus is another systemic condition associated with higher wound complications and infection rates.11,17,22–25 Hyperglycemia has been shown to impede wound healing by inhibiting both protocollagen and collagen synthesis, disrupting fibroblast proliferation, and slowing capillary ingrowth.22 The increased infection rate appears to be due to deficiencies in polymorphonuclear neutrophil function, including chemotaxis and phagocytosis.22,23,26,27
Patients taking corticosteroids have been reported to have a higher incidence of wound healing problems and infection.20,28 Direct effects of these medications include a reduction in collagen synthesis, vascular ingrowth, and downregulation of macrophage function, including migration to sites of inflammation and secretion of proteinases and collegenase.20,29 Longer duration of corticosteroid administration (>3 years) appears to be associated with increased wound complications.20 Patients on other immune-modifying chemotherapy agents may also be at increased risk. In particular, in patients with rheumatoid arthritis, methotrexate taken during the perioperative period may inhibit healing.26
Tobacco smoking has been shown to be the single most important risk factor for the development of postoperative complications, such as cardiopulmonary and wound healing problems, after hip and knee replacement; indeed, the proportion of smokers with wound complications was twice that of nonsmokers in one recent study.30 Smoking has also been shown to have a significant impact on flap survival.31 These effects are likely due to the potent peripheral vasoconstriction produced by nicotine, and other by-products of tobacco smoke.32 The inhibition of the dermal microcirculation has been shown to produce tissue hypoxia at a level known to be associated with poor wound healing.33 Cotinine, which is a metabolite of nicotine, has a much longer half-life (–19 hours vs. –120 minutes) and may be used as an effective marker of abstinence.32,34 Levels of cotinine exceeding back ground levels seen in non smokers would be expected to persist for 2.2 to 7.6 days.34 Cessation of smoking for several weeks prior to surgery should be encouraged, but even eliminating smoking in the perioperative period may produce significant advantages.31 Anemia has been cited as another factor that may contribute to wound complications. However, it appears that wound oxygen tension is the critical variable rather than absolute hemoglobin levels. Both angiogenesis and fibroblast function are inhibited by inadequate oxygen tension.14 The mild, normovolemic anemia that occurs routinely in postoperative patients may not impede wound healing.12,14,35,36 In patients with good cardiac and pulmonary function, a hematocrit as low as 15% to 18% may allow adequate healing.14 Poor wound oxygen tension is also associated with increased infection rates as wound hypoxia also appears to inhibit neutrophil bactericidal activity.14,37 Thus, it seems advisable to correct factors that contribute to low tissue perfusion, including a low hematocrit, dehydration, and peripheral vasoconstriction. Peripheral vascular disease should also be careful evaluated preoperatively.
Vascular surgery consultation should be sought for any patient with a history of claudication or prior bypass surgery. In addition, physical findings such as poor pedal pulses, venous stasis changes, or significant vascular calcifications noted on routine radiographic examination should prompt a complete evaluation. Furthermore, avoiding the use of an intraoperative tourniquet in any patient with a history of a prior ipsilateral lower extremity bypass or revascularization procedure is wise.
Local Factors Previous incisions and the status of the soft tissue about the knee should be carefully evaluated preoperatively. The age, location, length, and orientation of each incision, as well as its relationship to other incisions should be carefully considered. Prior surgical procedures have been associated with increased infection rates that may reflect problems with primary wound healing.18,21 Prior disruption of the dermal plexus and scarring down of the local soft tissue render subsequent incisions more likely to experience delayed healing. The possibility of flap necrosis also exists in patients with multiple anterior incisions. In particular, wide scars should be treated with caution as they may reflect significant disruption of the underlying vascular supply. In our experience, skin demonstrating local postradiation effects, including atrophy and adhesions, is perhaps the most concerning with little tolerance to subsequent surgical disruption. In general, a single transverse or a gently inclined, oblique incision may be crossed at a right angle with little concern. If a long, single anterior incision is present, it should be utilized unless it lies far medial or lateral. In circumstances where a prior vertical incision is significantly displaced from the midline, especially with short well-matured scars, a second vertical midline incision may be used if an adequate skin bridge of 2.5 to 5 cm can be maintained.38,39 If multiple vertical or mixed anterior incisions are present, especially in patients with systemic risk factors, the surgeon may be apprehensive about the ability of the soft tissues to heal primarily after the proposed surgery. In these cases, specialized techniques have been developed to help minimize wound healing complications. Unfortunately, specific criteria to help objectively identify those patients who require the use of these techniques have not been well defined. Instead, much of the decision making must be based upon experience. These techniques, described below in detail, include the use of prophylactic sham incisions, tissue expanders, and soft tissue flaps. In circumstances where these techniques are used, careful soft tissue management should still be used; in most cases, the most lateral prior incision should be incorporated in the exposure, as the predominant vascular supply is from the medial side.2,9,10
THE SHAM INCISION
The sham incision has been previously well described but in our practice is now rarely indicated as the development of tissue expander techniques has provided a superior alternative.40 However, sham incisions are interesting from a historical perspective, and represent a low-cost alterative to tissue expanders in patients who are unwilling to consider tissue expanders, or in health care settings where tissue expanders are unavailable. Although some vascular proliferation may result with the use of the sham incision technique, the main benefit of this technique is to identify those patients, where the wound will not heal prior to exposure of the knee joint and implantation of prosthetic components. In distinction, tissue expanders produce a significant neovascularization response in addition to physically stretching the skin that reduces the risk of poor wound healing.
The sham incision technique is performed approximately 2 weeks prior to the planned total knee procedure; the intended skin incision is made and extended through the subcutaneous tissue to the level of the extensor mechanism.40 Next, the incision is closed in the standard fashion and the wound observed. If the wound heals uneventfully, the intended knee surgery is then performed through the same incision. However, if wound healing problems occur, a soft tissue coverage procedure, such as a fasciocutaneous or muscle flap, is performed prior to the definitive knee surgery. The main advantage of this technique is that if wound problems occur, soft tissue procedures can be performed without placing the patient at risk for a potentially devastating deep periprosthetic joint infection. While the sham incision has proven useful in the past, it has been made redundant in our practices by the application of tissue expander techniques to the soft tissues about the knee.
TISSUE EXPANDERS
Tissue expansion techniques have been used extensively by plastic surgeons throughout the body. In these cases, expandable, saline-filled implants are inserted in the subcutaneous space and periodic inflation with saline that is injected through an access port is performed. Utilizing the concept of “stress relaxation,” the tissue overlying the expander is gradually stretched.41 However, the process involves more than a simple thinning of the dermis and subcutaneous fat as the epidermis also hypertrophies.42 The nature of the tissue also changes. In the epidermis, higher mitotic activity occurs, and in the dermis, more active fibroblasts, thicker elastic fibers, and vascular dilatation occur.42 During the expansion process, a distinct fibrous capsule develops around the tissue expander (Fig. 50-3) that can be useful at the time of closure. This capsule has a prominent vascular layer of dilated and new blood vessels (Fig. 50-4).42 Therefore, the expansion process not only produces additional tissue, but the physical forces behind expansion stimulate angiogenesis, which improves the healing capabilities of the tissue.43 It is because of these multiple benefits that tissue expanders have supplanted the use of sham incisions in our practices.
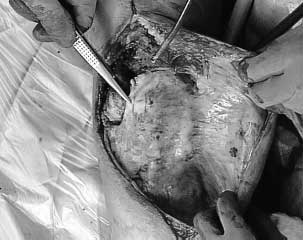
FIGURE 50-3. A thick fibrous capsule develops around the tissue expander during the expansion process.

FIGURE 50-4.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








