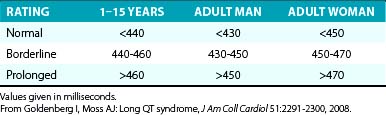Chapter 31 Ventricular Arrhythmias in Inherited Channelopathies
Ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT) is the initial rhythm recorded in 25% to 36% of witnessed cardiac arrests occurring at home, but in a much higher proportion (38% to 79%) of witnessed cardiac arrests occurring in a public setting.1 The majority of SCD events are associated with structural heart disease, with coronary artery disease and its complications being involved in up to 60% to 80% of cases, followed by other cardiomyopathies. However, in 10% to 20% of SCDs, no cardiac structural abnormalities are detectable. The lack of an apparent cause in many of those cases initially led to the classification as “sudden unexplained death syndrome” (SUDS) or “sudden infant death syndrome” (SIDS). Many of these are caused by primary electrical disorders, including long QT syndrome (LQTS), catecholaminergic polymorphic VT (CPVT), Brugada syndrome, and short QT syndrome (SQTS), as well as cases identified as “idiopathic VF” when the underlying cause often remains unknown.2
Long QT Syndrome
The LQTS is a rare inherited cardiac channelopathy with variable penetrance that is associated with an abnormally prolonged QT interval and an increased propensity to life-threatening ventricular arrhythmias in the presence of a structurally normal heart.3–5
The progressive unraveling of the molecular basis of LQTS has disclosed that whereas the autosomal dominant Romano-Ward syndrome depends on mutations affecting at least five genes encoding sodium (Na+) and potassium (K+) channels, the autosomal recessive Jervell and Lange-Nielsen syndrome depends on homozygous or compound heterozygous mutations of either one of the two genes encoding the subunits forming the channel conducting the slowly activating delayed rectifier K+ current (IKs). Being a recessive disease, the Jervell and Lange-Nielsen syndrome is far less common than the Romano-Ward syndrome.3,6,7
The initial molecular studies suggested that all genes linked to the LQTS phenotype encode for various subunits of cardiac ion channels. Subsequent findings, however, revealed that LQTS could also be caused by mutations of genes coding for channel-associated cellular structural proteins as well. Nonetheless, the concept that LQTS genes ultimately affect cardiac ion currents, either directly (ion channel mutations) or indirectly (modulators), still holds true.3
Epidemiology
There are no systematic studies on LQTS prevalence in the general population. A recent estimate of the prevalence of LQTS is 1:2000 based on the results of genetic screening in families and the incidence of compound heterozygotes (i.e., persons with two mutations). However, the clinical disease is less common (approximately 1 in 5000) because most mutation carriers remain asymptomatic.8 The usual mode of inheritance is autosomal dominant, with the exception of the autosomal recessive Jervell and Lange-Nielsen type.6
Clinical Presentation and Natural Course
Romano-Ward Syndrome
Most information regarding the clinical features of LQTS have been derived from analysis of data from large series of LQTS patients, the largest of which is the International LQTS Registry. LQTS probands are diagnosed at an average age of 21 years. The clinical course of patients with LQTS is variable, owing to incomplete penetrance. It is influenced by age, genotype, gender, environmental factors, therapy, and possibly other modifier genes. At least 37% of individuals with the LQT1 phenotype, 54% with the LQT2 phenotype, and 82% with the LQT3 phenotype remain asymptomatic and many are referred for evaluation because of the diagnosis of LQTS of a family member or the identification of long QT interval on surface electrocardiogram (ECG) obtained for unrelated reasons.5
Syncope is the most frequent symptom, occurring in 50% of symptomatic probands by the age of 12 years, and in 90% by the age of 40 years. The incidence of syncope in LQTS patients is approximately 5% per year, but can vary depending on the LQTS genotype. On the other hand, the incidence of SCD is much lower, approximately 1.9% per year. Nonfatal events (syncope and aborted cardiac arrest) in LQTS patients remain the strongest predictors of subsequent LQTS-related fatal events. The overall risk of subsequent SCD in an LQTS patient who has experienced a previous episode of syncope is approximately 5% per year.9
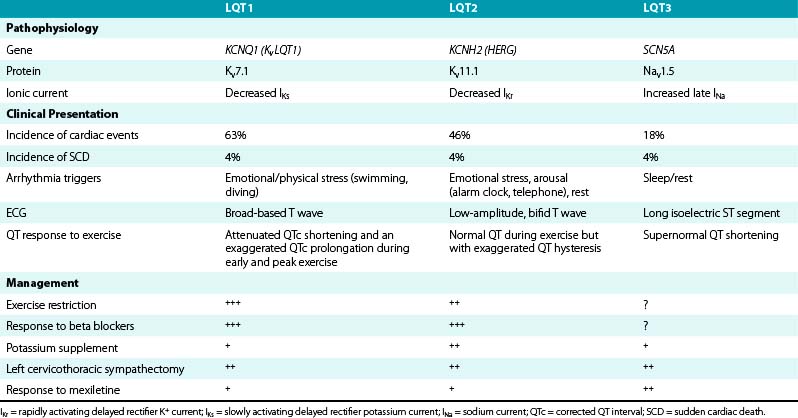
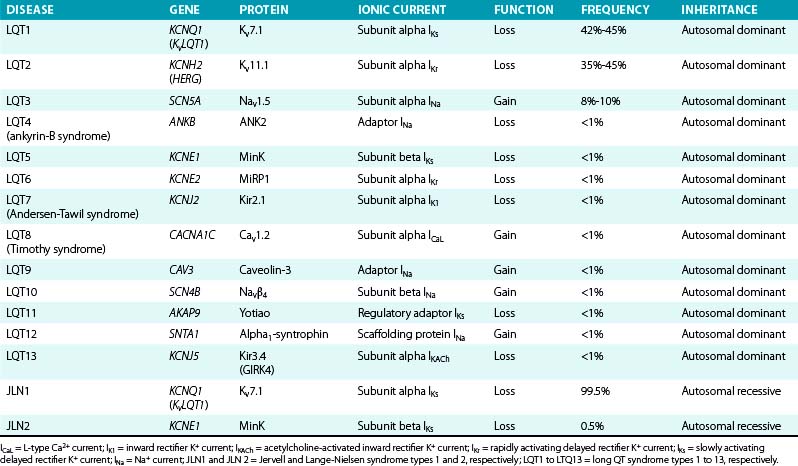
LQT1, LQT2, and LQT3 comprise more than 90% of all genotyped LQTS cases. LQT1 is the most frequent genetic form of LQTS, accounting for 42% to 45% of genotyped LQTS cases. LQT2 is the second most prevalent form of the disease and accounts for 35% to 45% of genotyped LQTS cases (Table 31-1).5,10
Risk and lethality of cardiac events among untreated individuals are strongly influenced by the genotype. The frequency of cardiac events is significantly higher among LQT1 (63%) and LQT2 (46%) patients than among patients with the LQT3 genotype (18%). However, the likelihood of dying during a cardiac event is significantly higher among LQT3 patients (20%) than among those with the LQT1 (4%) or the LQT2 (4%) genotype.10–12
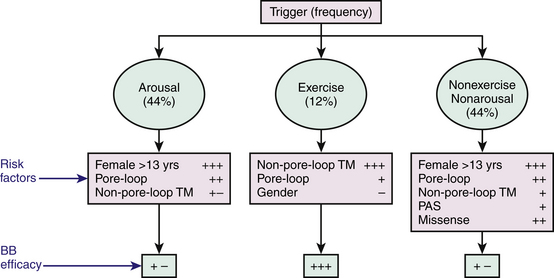
Cardiac events (syncope, cardiac arrest, SCD) in LQTS patients do not occur at random; the factors precipitating cardiac events seem to be specific for each genetic variant. LQT1 patients present an increased risk during physical or emotional stress (90%), and only 3% of the arrhythmic episodes occur during rest/sleep. Swimming and diving appear as highly specific triggers in LQT1 patients. LQT2 patients are at higher risk for lethal events during arousal (44%), but are also at risk during sleep and at rest (43%). Only 13% of cardiac events occur during exercise. Cardiac events in LQT2 patients are typically associated with arousal and auditory stimulation. In fact, the triggering of events by startling, sudden awakening, or sudden loud noises (such as a telephone or alarm clock ring) is virtually diagnostic of LQT2. Notably, individual factors such as gender, location and type of mutation, and QTc prolongation appear to be associated with trigger-specific events; female adolescents with LQT2 appear to experience a greater than ninefold increase in the risk for arousal-triggered cardiac events compared with male adolescents in the same age group. In contrast, gender does not seem to be a significant risk factor for exercise-triggered events among carriers of the same genotype (Fig. 31-1).13
On the other hand, LQT3 patients experience cardiac events largely while asleep or at rest (65%) without emotional arousal, and only occasionally during exercise (4%). Notably, the majority of patients continue to experience their cardiac events under conditions similar to their first classified event.10,11
The effect of gender on outcome is age-dependent, with boys being at higher risk than girls during childhood and early adolescence, but no significant difference in gender-related risk being observed between 13 and 20 years. The gender-related risk reverses afterward, and female patients maintain higher risk than male patients throughout adulthood.14
The genotype can potentially affect the clinical course of the LQTS and modulate the effects of age and gender on clinical manifestations.15 Although the three major LQTS genotypes (LQT1, LQT2, or LQT3) are associated with similar risks for life-threatening cardiac events in children and adolescents after adjustment for clinical risk factors (including gender, QTc duration, and time-dependent syncope), the risk for cardiac events is augmented in LQT2 women aged 21 to 40 years and in LQT3 patients greater than 40 years of age. The risk of syncope and SCD decreases during pregnancy but increases in the postpartum period, especially among LQT2 women.10–12
Jervell and Lange-Nielsen Syndrome
Patients with Jervell and Lange-Nielsen syndrome have a more severe cardiac phenotype than those with Romano-Ward syndrome. Complete loss of IKs in hair cells and endolymph of the inner ear results in congenital bilateral sensorineural deafness. Patients begin to experience cardiac events very early in life; 15% suffer a cardiac event during the first year of life, 50% by age 3 years, and 90% by age 18 years. In untreated patients, approximately 50% die of ventricular arrhythmias by the age of 15 years. Furthermore, SCD occurs in more than 25% of patients despite medical therapy.6
The conditions that trigger the cardiac events are, overall, very similar to those described for LQT1. Up to 95% of events occur during sympathetic activation (exercise and emotions), and only 5% of the events occur at rest or during sleep.6
Andersen-Tawil Syndrome
Andersen-Tawil syndrome (LQT7) is a rare autosomal dominant disorder caused by mutations of the gene KCNJ2, which encodes the inward rectifier potassium channel, Kir2.1. This syndrome is characterized by a triad of a skeletal muscle phenotype (periodic paralysis caused by abnormal muscle relaxation), a cardiac phenotype (borderline or mildly prolonged QT interval, prominent U waves, and adrenergically mediated ventricular arrhythmias), and distinctive skeletal dysmorphic features (low-set ears, ocular hypertelorism, small mandible, fifth-digit clinodactyly, syndactyly, short stature, scoliosis, and a broad forehead).16–18
Affected individuals present initially with either periodic paralysis or cardiac symptoms (palpitations, syncope, or both) in the first or second decade. The arrhythmias displayed by affected patients are generally more benign compared with other types of LQTS and rarely degenerate into hemodynamically compromising rhythms like torsades de pointes, as ultimately evidenced by the lack of SCD cases so far.17 Intermittent weakness occurs spontaneously, or may be triggered by prolonged rest or rest following exertion; however, the frequency, duration, and severity of symptoms are variable between and within affected individuals, and are often linked to fluctuations in plasma potassium levels. Mild permanent weakness is common.
Timothy Syndrome
Extracardiac features include cutaneous syndactyly (variably involving the fingers and toes), which is observed in almost all patients. Facial findings (observed in approximately 85% of individuals) include low-set ears, flat nasal bridge, thin upper lip, small upper jaw, small, misplaced teeth, and round face. Neuropsychiatric involvement occurs in approximately 80% of individuals and includes global developmental delays and autism spectrum disorders.
Electrocardiographic Features
QT Interval Measurement
QT interval is the body surface representation of the duration of ventricular depolarization and subsequent repolarization. Any deviation or dispersion of either depolarization (e.g., bundle branch block) or repolarization (e.g., prolongation or dispersion of the action potential duration) prolongs the QT interval.19
An accurate measurement of the QT interval is important for the diagnosis of LQTS. A 12-lead ECG tracing at a paper speed of 25 mm/sec at 10 mm/mV is usually adequate to make accurate measurements of the QT interval. The QT interval is measured as the interval from the onset of the QRS complex, that is, the earliest indication of ventricular depolarization, to the end of the T wave, that is, the latest indication of ventricular repolarization. The QT interval is measured in all ECG leads where the end of the T wave can be clearly defined (preferably leads II and V5 or V6), with the longest value being used. The end of the T wave is the point at which the descending limb of the T wave intersects the isoelectric line. Three to five consecutive cardiac cycles are taken to derive average values for R-R, QRS, and QT intervals.5,20–23
When the end of the T wave is indistinct, or if a U wave is superimposed or inseparable from the T wave, it is recommended that the QT be measured in the leads not showing U waves (often aVR and aVL) or that the downslope of the T wave be extended by drawing a tangent to the steepest proportion of the downward limb of the T wave until it crosses the baseline (i.e., the T-P segment). Nonetheless, it should be recognized that defining the end of the T wave in these ways might underestimate the QT interval.22 Some investigators advocate measurement of both the QT interval and the QTU interval (with the latter measurement taken to the end of the U wave as it intersects the isoelectric line) because the QTU interval probably reflects the total duration of ventricular depolarization.20
The highest diagnostic and prognostic value in LQTS families has been observed for QTc in leads II and V5 of the 12-lead ECG. Thus, QTc should be obtained in one of these leads if measured in only one ECG lead. However, other leads presented with similar diagnostic (aVR) or prognostic (V2/V3) value alone, and, in general, the lead with the longest QT interval is used for measurement.23
QT Interval Correction for Gender
The QT interval shortens after puberty in men but not women, resulting in a longer QT in women than in men. The reported gender difference in various studies varies from 6 to 10 milliseconds in older age groups and from 12 to 15 milliseconds in younger adults. Overall, the gender difference in the rate-corrected QT interval becomes small after 40 years of age and practically disappears in older men and women. Separate gender- and age-specific QT adjustment formulas have been proposed to accommodate these differences.22
QT Interval Correction for Heart Rate
Because the heart rate (R-R cycle length) is the primary modifier of ventricular action potential, QT interval measurements must be corrected for the individual’s R-R interval (QTc) to allow for comparisons. Various correction formulas have been developed (Table 31-2), the most widely used being the formula derived by Bazett in 1920 from a graphic plot of measured QT intervals in 39 young subjects. The Bazett correction, however, performs less well at high and low heart rates (undercorrects at fast heart rates and overcorrects at slow heart rates).20 Therefore, it was recommended by the American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society (AHA/ACC/HRS) in 2009 that linear regression functions rather than the Bazett formula be used for QT rate correction. In addition to the Bazett formula, many other correction formulas, such as the Framingham-Sagie, Fridericia, Hodges, and Nomogram-Karjalainen formulas, have been proposed.5,21,22
TABLE 31-2 Formulas for Heart Rate Correction of the QT Interval
| Bazett | QTcB = QT/(R-R interval)1/2 (all intervals in seconds) |
| Framingham-Sagie | QTcFa = QT + 154(1 − 60/heart rate) |
| Fridericia | QTcFi = QT/(R-R interval)1/3 |
| Hodges | QTcH = QT + 1.75(heart rate − 60) |
| Nomogram-Karjalainen | QTcN = QT + nomogram correction factor |
In a recent report, the accuracy of five different QT correction formulas was evaluated for determining drug-induced QT interval prolongation. The Bazett correction formula provided the most marked QTc variations at heart rates distant from 60 beats/min. The Fridericia formula was found to overestimate QTc at faster heart rates, being more reliable at slower heart rates. Conversely, the Hodges, Nomogram, and Framingham formulas demonstrated less QTc variability over the whole range of the investigated heart rates and seemed to be similarly satisfactory at heart rates of up to 100 beats/min. Among them, the Hodges method, followed by the Nomogram-Karjalainen method, appeared to be the most accurate in determining the correct QTc and subsequently in guiding clinical decisions.21
Importantly, there exists a substantial interindividual variability of the QT/R-R relationship, which represents the relationship between QT duration and heart rate. In contrast, a high intraindividual stability of the QT/R-R pattern has been shown, suggesting that a genetic component might partly determine individual QT length. Therefore, population-based and averaged QT correction cannot accurately predict a normal QT interval at a given R-R interval in a given patient. Individual-specific QT/R-R hysteresis correction in combination with individualized heart rate correction can potentially reduce intrasubject QTc variability.19,24
QT Interval Correction to QRS Duration
The QT interval prolongs in ventricular conduction defects, and an adjustment for QRS duration becomes necessary. This can be accomplished best by incorporating QRS duration and R-R interval as covariates into the QT adjustment formula or by using the JT interval (QT duration minus QRS duration). If the JT interval is chosen, normal standards established specifically for the JT interval should be used. QT and JT adjustment formulas have recently been introduced for use in the setting of prolonged ventricular conduction. With confirmation, they may be incorporated into automated algorithms to provide appropriate correction factors.22
QT Interval Prolongation
The diagnosis of QT interval prolongation can be challenging because of the difficulty in defining the “end” of the T wave and the need for correction for heart rate, age, and gender. This is further complicated by the lack of linear behavior of the Bazett formula at slower and faster heart rates as well as the arbitrary definition of the gender-based diagnostic cut-off values that define an abnormally prolonged QTc (QTc of 450 milliseconds for men and QTc of 460 milliseconds for women; Table 31-3).
Furthermore, no single QTc value separates all LQTS patients from healthy controls. The QT interval is subject to large variations even in healthy individuals, and substantial overlap exists with QTc values obtained from LQTS mutation carriers in the range between 410 and 470 milliseconds. In up to 40% of LQTS patients QTc intervals fall in the normal range. Nevertheless, QTc values greater than 470 milliseconds in men and greater than 480 milliseconds in women are practically never seen among healthy individuals (especially when their heart rate is 60 to 70 beats/min), but LQTS cannot be excluded merely by the presence of a normal QTc interval.25 Therefore, unless excessive QTc prolongation (>500 milliseconds, corresponding to the upper quartile among affected genotyped individuals) is present, the QTc interval should always be evaluated in conjunction with the other diagnostic criteria. In the case of borderline QTc prolongation, serial ECG and 24-hour Holter recordings can potentially assist in establishing QT prolongation, as can various challenge tests.7
Of note, a considerable variability in QTc interval duration can be observed in patients with LQTS when serial ECGs are recorded during follow-up. This time-dependent change in QTc duration is an important determinant of the phenotypic expression of the disease. Up to 40% of patients with LQTS will have QTc greater than 500 milliseconds at least once during long-term follow-up, but only 25% will have that degree of QT prolongation during their initial evaluation. The maximal QTc duration measured at any time before age 10 years was shown to be the most powerful predictor of cardiac events during adolescence, regardless of baseline, mean, or most recent QTc values. In addition, the QT interval normally may exhibit individual variations during the day.25,26
T Wave Morphology
ST-T wave abnormalities are common among LQTS patients, and some of the ST-T anomalies are characteristic for a specific genotype. Patients with LQT1 commonly exhibit a smooth, broad-based T wave that is present in most leads, particularly evident in the precordial leads. The T wave generally has a normal to relatively high amplitude and often no distinct onset. LQT2 patients generally present with low-amplitude T waves, which are notched or bifid in approximately 60% of carriers. The bifid T wave can be confused with a T-U complex; however, unlike the U waves, the bifid T waves are usually present in most of the 12 ECG leads. LQT3 patients often show late-onset, narrow, peaked, and/or biphasic T waves with a prolonged isoelectric ST segment. Occasionally, the T wave is peaked and asymmetrical with a steep downslope. These ST-T wave patterns can be seen in 88% of LQT1 and LQT2 carriers and in 65% of LQT3 carriers. However, exceptions are present in all three genotypes, and the T wave pattern can vary with time, even in the same patient with a specific mutation.27,28
No specific T wave pattern has been suggested in the LQT5 and LQT6 syndromes. T-U wave abnormalities such as biphasic T waves following long pauses like those found in the LQT2 syndrome are commonly observed in the LQT4 syndrome. Enlarged U waves separated from the T wave are reported to be characteristic ECG features in the LQT7 syndrome. Severe QT interval prolongation and macroscopic T wave alternans can be observed in LQT8.27
Despite the initial enthusiasm in achieving a genotype-phenotype correlation for specific LQT-associated genes, this approach failed to provide a high diagnostic yield because of the frequent exceptions in T wave morphology presentation. Therefore, other T wave parameters, such as duration, amplitude, asymmetry, and flatness, as well as the T wave peak–to–T wave end interval, have been used as highly specific quantitative descriptors (see below).28
Torsades De Pointes
The tachycardia rate typically is in the range of 150 to 300 beats/min. In many cases, torsades de pointes is a self-limiting arrhythmia that spontaneously dies out after a few tens of cycles; however, most patients experience multiple episodes of the VT occurring in rapid succession. Only in a minority of cases does torsades de pointes degenerate into VF, which almost without exception leads to SCD if immediate rescue intervention is unavailable.7
Dispersion of Repolarization
In experimental models of the LQTS, prolonged repolarization, transmural dispersion of repolarization, and early afterdepolarizations (EADs) are the three EP components linked to the genesis of torsades de pointes. Prolongation of the action potential duration (and QT interval) per se is not pathogenic, as demonstrated by the fact that a homogeneous action potential duration prolongation (such as occurs following amiodarone therapy) fails to generate reentry. Several ECG indices have been proposed in recent years as noninvasive surrogates for transmural dispersion of repolarization. Transmural dispersion of repolarization arises from repolarization heterogeneity that exists between the epicardial and putative midmyocardial (M) cells that lie toward the endocardium of the left ventricular (LV) wall. These midmyocardial cells are especially sensitive to a repolarization challenge and exhibit significant prolongation of the action potential duration compared with other transmural cell types.7,29,30
Although some controversy exists, some experimental models suggest that the peak of the T wave coincides with the end of epicardial repolarization (the shortest action potentials), whereas the end of the T wave coincides with the end of repolarization of the M cells (the longest action potentials). Hence, the interval from the peak of the T wave to the end of the T wave (Tpeak-to-end) in each surface ECG lead has been proposed as an index of transmural repolarization, which can potentially be a more sensitive predictor of arrhythmogenic risk than the QT interval, because the latter represents the total duration of electrical ventricular activation) and not necessarily the dispersion of transmural repolarization.7,29,30
Although changes in the Tpeak-to-end interval can potentially show the dynamicity of the transmural dispersion of repolarization in clinical settings in LQTS patients, the role of measuring the Tpeak-to-end interval in these patients is not clearly defined yet. In fact, the normal value of the Tpeak-to-end interval on the ECG has not been established. Nonetheless, an interval of more than 100 milliseconds is uncommon in normal subjects compared with that in subjects with LQTS (9% versus 55%).30
LQT2 exhibits a larger-degree transmural dispersion of repolarization, as measured by the Tpeak-to-end interval, compared with LQT1 and normal hearts. In fact, the Tpeak-to-end interval has been proposed as a diagnostic criterion in differentiation between LQT2 and LQT1 patients. Additionally, whereas LQT1 patients and normal subjects demonstrate stable Tpeak-to-end intervals independent of heart rates, LQT2 patients exhibit a trend to decreased Tpeak-to-end intervals at fast heart rates and to increased Tpeak-to-end durations at slow heart rates. In both LQT1 and LQT2 patients, the longest Tpeak-to-end intervals are associated with sudden changes in the heart rate trend. Importantly, the magnitude of transmural dispersion of the repolarization interval does not seem to differ between asymptomatic and symptomatic patients in either LQT1 or LQT2. Conversely, symptomatic LQT2 patients exhibit a trend toward longer QT intervals than do asymptomatic patients.29
On ambulatory monitoring, transmural dispersion of repolarization, measured as Tpeak-to-end intervals during normal daily activities, appears to be greater in LQT2 than in LQT1 patients. LQT1 patients exhibit abrupt increases in Tpeak-to-end intervals at elevated heart rates, whereas LQT2 patients exhibit increases in transmural dispersion of repolarization at a much wider range of rates. In addition, beta-adrenergic stimulation (exercise or epinephrine infusion) increases transmural dispersion of repolarization in both LQTS models, transiently in LQT2 and persistently in LQT1.29
An alternate approach to determine repolarization heterogeneity is provided by the QT interval dispersion. The QT dispersion index is obtained by the difference between the maximal and minimal QT intervals (QTmax − QTmin) measured on a 12-lead ECG. It reflects the spatial heterogeneity of myocardial refractoriness more accurately than single QT values. Visualization of the differences in QT interval in the different ECG leads is facilitated by the display of a suitable subset of temporally aligned simultaneous leads with a slight separation on the amplitude scale.22 However, QT interval dispersion measurements are subject to similar shortcomings encountered with the QT interval assessment, as a large overlap between affected and healthy individuals is observed.7 Further, accurate measurement on the scalar ECG at a paper speed of 25 mm/sec is difficult. The same caveat applies to measuring Tpeak-to-end.
The ratio of the amplitudes of the U and T waves has been suggested as the clinical counterpart of EADs, and a progressive increase in this ratio was found to precede the onset of torsades de pointes in an experimental model of LQTS. In addition, the increment in U wave amplitude after a premature ventricular complex (PVC) has been suggested as a marker for arrhythmia risk in “pause-dependent” LQTS. In patients with bifid T waves, some investigators used the late component of the T wave, rather than the U wave. The diurnal maximal ratio between late and early T wave peak amplitude correlates with a history of LQTS-related symptoms better than the baseline QTc interval in both LQT1 and LQT2 patients. In LQT1 and LQT2 patients, diurnal distributions of maximal T2-to-T1 wave amplitude ratios are similar to reported corresponding distributions of cardiac events, and can potentially be used as a predictor of the arrhythmia risk for asymptomatic patients with known type 1 or 2 LQT genotype.31
Diagnosis of the Long QT Syndrome
The LQTS is a clinical diagnosis, primarily based on the clinical presentation, personal and family history, and ECG findings. A detailed family history of syncope and SCD is essential, not only in first-degree relatives (mother, father, siblings, children), but also in more distant relatives. Importantly, family history should also be investigated for other potential manifestations of malignant arrhythmias that might not have been classified as cardiac in origin, such as drowning, death while driving, epileptic seizures, as well as SIDS. Data on comorbidities in evaluated individuals or family members (such as congenital deafness) should also be acquired. Clinical features such as triggers of syncope and specific QT morphological attributes in patients in whom the clinical diagnosis has been made can suggest the affected gene in 70% to 90% of patients.5,32
The QTc interval is the most common standardized parameter used for diagnosing LQTS and quantifying ventricular repolarization. As noted, QTc values greater than 470 milliseconds in men and greater than 480 milliseconds in women are practically never seen among healthy individuals (especially at heart rates of 60 to 70 beats/min) and are considered diagnostic of LQTS (as long as acquired forms of LQTS are excluded) even in asymptomatic subjects and those having a negative family history. When a prolonged QTc is observed during faster heart rates (>90 beats/min), it is important to repeat the ECG once the heart rate slows down, to minimize the error that can potentially be introduced by heart rate correction formulas.25
Additionally, LQTS can be suspected in individuals with QTc values exceeding 450 milliseconds for men and exceeding 460 milliseconds for women; these subjects are considered to have “high probability for LQTS” if they have a history of syncope and familial SCD. On the other hand, LQTS is very unlikely among men with QTc less than 390 milliseconds and among women with QTc less than 400 milliseconds.25
Importantly, a significant proportion (20% to 40%) of patients with genetically proven LQTS have normal or borderline QTc measurements at rest (“concealed” LQTS). Therefore, the diagnosis of LQTS should not be excluded based solely on a QTc interval in the normal range (400 to 450 milliseconds), and additional testing is indicated whenever the clinical history requires exclusion of LQTS. In this setting, obtaining a resting ECG periodically sometimes can uncover an abnormal prolongation of the QTc interval, given the considerable day-to-day variability in QTc of patients with LQTS. Additionally, reviewing the ECGs of all family members can be valuable, because some family members can have obvious QT prolongation. Several additional clinical tests and tools have been used in the clinical evaluation of LQTS, including ambulatory ECG recordings, exercise stress testing, epinephrine QT stress testing, and genetic testing. Although these diagnostic tools can all contribute to identifying patients with LQTS, a “gold standard” diagnostic tool is still lacking. Invasive EP testing is generally not useful in the diagnosis of LQTS.33,34
Clinical Scoring Systems
When the diagnosis is not clear, two clinical scoring systems have been developed to enhance the diagnostic reliability of clinical parameters and to estimate the probability of LQTS: the Keating criteria and the Schwartz score. The Keating criteria are a binary combination of QTc values with LQTS-related symptoms. According to these criteria, individuals are affected if they have a QTc interval greater than 470 milliseconds even in the absence of symptoms, or if they have typical symptoms with a QTc interval greater than or equal to 450 milliseconds. The Schwartz score incorporates ECG features in combination with personal clinical history and family history. Scores were arbitrarily divided into three probability categories, providing a quantitative estimate of the risk for LQTS (Table 31-4).35,36
TABLE 31-4 Diagnostic Criteria for the Long QT Syndrome
| FINDING | SCORE |
|---|---|
| ECG* | |
| QTc† ≥ 480 msec | 3 |
| QTc† = 460-470 msec | 2 |
| QTc† = 450 msec (in men) | 1 |
| Torsades de pointes‡ | 2 |
| T wave alternans | 1 |
| Notched T wave in three leads | 1 |
| Low heart rate for age§ | 0.5 |
| Clinical History | |
| Syncope with stress‡ | 2 |
| Syncope without stress‡ | 1 |
| Congenital deafness | 0.5 |
| Family History¶ | |
| Family members with definite LQTS | 1 |
| Unexplained SCD in immediate family members <30 yr of age | 0.5 |
Scoring: 1 point, low probability of LQTS; 2 or 3 points, intermediate probability of LQTS; 4 or more points, high probability of LQTS.
LQTS = long QT syndrome; SCD = sudden cardiac death.
* Findings in the absence of medications or disorders known to affect these ECG findings.
† The corrected QT interval (QTc) is calculated by the Bazett formula.
‡ Torsades de pointes and syncope are mutually exclusive.
§ Resting heart rate below the second percentile for age.
¶ The same family member cannot be counted in both categories.
Modified from Goldenberg I, Moss AJ: Long QT syndrome, J Am Coll Cardiol 51:2291-2300, 2008.
When applied to family members of positively genotyped patients, these systems demonstrated excellent high specificity (99%) but very low sensitivity (19% for the Schwartz “high probability” score and 36% for the Keating criteria), and severely underdiagnosed disease carriers (false negatives). Significant underdiagnosis was also found among probands, despite the fact that probands are generally more seriously affected than their relatives. A high probability of LQTS (Schwartz criteria) was found only in 57% of probands with a confirmed molecular diagnosis. The performance of QTc interval measurement alone (with a cut-off value of 430 milliseconds) is superior to the Schwartz and Keating criteria when DNA testing is available for the confirmation of disease carriership, as it has far better sensitivity (72%) while retaining reasonable specificity (86%). Thus, in families with known causal gene mutations, genetic analysis is the method of choice to identify the relatively high proportion of silent carriers of disease-causing mutations.35,36
Ambulatory Cardiac Monitoring
Holter monitoring is not sufficiently well standardized to serve in the primary assessment for ventricular repolarization analysis, and only rarely will show spontaneous arrhythmias in LQTS patients. However, this method can sometimes be used for the detection of extreme QT interval events that occur infrequently during the day. Characteristic T wave changes may be revealed during sleep or following post-extrasystolic pauses.25,32
Exercise Stress Testing
Attenuated QTc shortening and an exaggerated QTc prolongation during early and peak exercise are characteristic of LQT1. As will be discussed later, genetic mutations in LQT1 result in reduction of the amplitude of IKs, one of the dominant K+ currents responsible for repolarization especially at rapid heart rates (during sympathetic stimulation). Attenuation of IKs results in failure of the QT to adapt (i.e., shorten) in response to increasing heart rate. Unlike LQT1 patients, normal subjects, LQT2 patients, and LQT3 patients decrease their respective QTc intervals from rest at peak exercise. A maladaptive paradoxical QTc prolongation during the recovery phase (QTc >460 milliseconds or a ΔQTc [QTc at 3 minutes of recovery minus the baseline supine QTc] >30 milliseconds) was found to distinguish patients with either manifest or concealed LQT1 from normal subjects and those with LQT2 and LQT3 genotypes.37
In contrast, patients with LQT2 mutations have normal QT shortening and minimal QTc prolongation during exercise, but they characteristically demonstrate an exaggerated QT hysteresis compared with LQT1 patients and normal subjects. QT hysteresis is normally measured by comparing the QT intervals during exercise versus the recovery period at comparable heart rates (e.g., when the heart rate accelerates to approximately 100 beats/min during early exercise and 1 to 2 minutes into the recovery phase, when the heart rate typically decelerates to approximately 100 beats/min). In LQT2 patients, the QT fails to shorten at these intermediate heart rates in early exercise because of attenuated IKr (rapidly activating delayed rectifier potassium current; a so-called IKr zone). This is followed by recruitment of the unimpaired, sympathetically responsive IKs, resulting in appropriate QT shortening at faster heart rates through to peak exercise, which persists into the recovery phase. This consequently leads to an exaggerated QT difference between exercise and recovery at comparable heart rates, which is manifested as increased QT hysteresis.34,38
The LQT3 phenotype is characterized by a constant shortening of the action potential duration (and QT interval) with exercise because of stimulation of the intact IKs channel and augmentation of a late inward Na+ current.38
Postural and exercise-induced QTc prolongation and QT hysteresis can be attenuated with beta blockade; therefore, beta blocker therapy should be discontinued before exercise testing. Additionally, exercise treadmill testing can also reveal the characteristic T wave morphology in patients with LQT1 and LQT2 syndromes.7
Importantly, induction of arrhythmias during exercise is very rare in LQTS patients. Exercise-induced ventricular ectopy exceeding isolated PVCs is observed in less than 10% of patients.7,32 The presence of exercise-induced ventricular ectopy beyond single, isolated PVCs must prompt intense evaluation because it was found to have a positive predictive value exceeding 90% for the presence of significant cardiac pathology. However, CPVT, rather than LQTS, is the far more likely diagnosis.39
Epinephrine QT Stress Test
Catecholamine provocation testing can help diagnose patients with concealed LQT1, with a positive predictive value approaching 75% and a negative predictive value of 96%. Furthermore, epinephrine provocation testing was found to be a powerful test to predict the genotype of LQT1, LQT2, and LQT3 syndromes.32,38,40
Two major protocols have been developed for epinephrine infusion. Using the “escalating-dose infusion protocol,” epinephrine infusion is initiated at 0.025 µg/kg/min and then increased sequentially every 10 minutes to 0.05, 0.1, and 0.2 µg/kg/min. The 12-lead ECG is continuously recorded during sinus rhythm under baseline conditions and during epinephrine infusion. The QT interval is measured 5 minutes after each dose increase. Epinephrine infusion should be stopped for systolic blood pressure greater than 200 mm Hg, nonsustained VT or polymorphic VT, frequent PVCs (>10 per minute), T wave alternans, or patient intolerance. A paradoxical QT interval response (prolongation of the absolute QT interval of ≥30 milliseconds) during low-dose epinephrine infusion provides a presumptive clinical diagnosis of LQT1, with a positive predictive value of 75%. The diagnostic accuracy can be reduced in patients receiving beta blockers.38
Using the “bolus and infusion protocol,” an epinephrine bolus (0.1 µg/kg) is administered and immediately followed by continuous infusion (0.1 µg/kg/min) for 5 minutes. The QT interval is measured 1 to 2 minutes after the start of epinephrine infusion when the R-R interval is the shortest (which represents the peak epinephrine effect) and 3 to 5 minutes after the start of epinephrine infusion (which represents the steady-state epinephrine effect).40
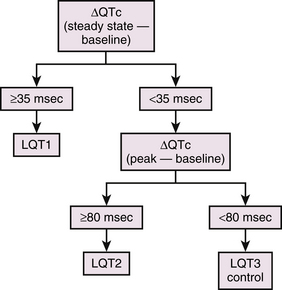
During the epinephrine test, patients with LQT1 manifest prolongation of the QTc at the peak of the epinephrine effect, which is maintained under steady-state conditions of epinephrine. In contrast, epinephrine prolongs the QTc more dramatically at the peak of epinephrine infusion in LQT2 patients, but the QTc returns to baseline levels under steady-state conditions. A much milder prolongation of QTc at the peak of epinephrine has been described in LQT3 patients and in healthy subjects, and it returns to the baseline levels under steady-state conditions. A subject is considered to have an LQT1 response if the QTc increase in the peak phase is greater than 35 milliseconds and is maintained throughout the steady-state phase (Fig. 31-2). LQT2 response is likely if the peak QTc increase of greater than 80 milliseconds is not maintained in the steady-state phase. In one report, the sensitivity and specificity of the epinephrine test to differentiate LQT1 from LQT2 were 97% and 96%, those from LQT3 were 97% and 100%, and those from healthy subjects were 97% and 100%, respectively, when ΔQTc greater than 35 milliseconds at steady state was used. The sensitivity and specificity to differentiate LQT2 from LQT3 or healthy subjects were 100% and 100%, respectively, when ΔQTc greater than 80 milliseconds at peak was used.40
The escalating-dose infusion protocol is generally better tolerated by the patient and carries a lower incidence of false-positive responses. On the other hand, the bolus and infusion protocol offers the ability to monitor the temporal course of the epinephrine response at peak dose (during the bolus) and during steady state (during the infusion), which is particularly important in individuals with LQT2 in whom transient prolongation of the uncorrected QT interval can occur, followed by subsequent shortening.38
Genetic Testing
Although the diagnosis of LQTS frequently can be certain based on clinical diagnostic measures, in which setting molecular screening may not be necessary, genetic testing can still be of value; identification of the specific gene affected (or the site of the mutation within the gene) can potentially guide therapeutic choice and enhance risk stratification. Importantly, identification of the disease-causing mutation in the proband provides the ability to easily identify affected family members and implement lifestyle adjustment and presymptomatic treatment, and is thereby potentially lifesaving. Furthermore, genetic testing may be important in the identification of concealed LQTS, because a significant minority (25% to 50%) of individuals with genetically proven LQTS have a nondiagnostic QTc.3,5,7,32
Genetic testing is a powerful tool to identify patients with LQTS. Yet, it remains expensive and unavailable to many centers. Depending on the stringency of clinical phenotype assessment, the yield for positive genetic results in LQTS ranges from 50% to 78%, and is highest among tested individuals with the highest clinical probability (i.e., those with longer QTc intervals and more severe symptoms). The remaining probands with a strong clinical probability of LQTS will have a negative genetic test result, probably because of technical difficulties with genotyping, noncoding variants, or as yet unidentified disease-associated genes. Therefore, a negative genetic test in a subject with clinical LQTS (i.e., genotype-negative/phenotype-positive LQTS) does not provide a basis to exclude the diagnosis.3,32–34
There is also the potential for false-positive results; genetic testing may identify novel mutations of unclear significance, which could represent normal variants, and require validation and further analysis (e.g., linkage within a family or in vitro studies).32–34
Currently, comprehensive or LQT1 to LQT3 (KCNQ1, KCNH2, and SCN5A)-targeted LQTS genetic testing is recommended for symptomatic patients with a strong clinical index of suspicion for LQTS as well as for asymptomatic patients with QT prolongation (QTc ≥480 milliseconds [prepuberty] or ≥500 milliseconds [adults]) in the absence of other clinical conditions that might prolong the QT interval.41
Genetics of the Long QT Syndrome
To date, more than 500 mutations of 13 different genes responsible for a hereditary form of LQTS have been identified (Table 31-5), with the majority of the known mutations located in the first three: LQT1 (KCNQ1) mutations account for 42% to 45% of genetically positive LQTS, LQT2 (KCNH2) for 35% to 45%, and LQT3 (SCN5A) for 8% to 10%.
Genetic analysis reveals two or more mutations in 5% to 10% of LQTS patients with clinical phenotypes of Romano-Ward syndrome. These compound mutations (so-called double hits) appear to be associated with a more severe phenotype than that associated with a single hit.42
Most reported mutations are in coding regions, although noncoding mutations (resulting in the loss of allele expression) have also been described. Most LQTS families have their own mutations, which are often termed “private” mutations.32
The majority of LQTS cases are caused by heterozygous disease; thus, mutations causing abnormalities in channel coassembly or trafficking result in up to 50% maximal reduction in the number of functional channels (haplotype insufficiency), because the gene product from the healthy allele remains intact. On the other hand, mutations that abolish channel function while preserving subunit assembly can result in dominant-negative suppression of the healthy allele as well, causing a more severe reduction (up to 94%) of the total amount of functional protein (dominant-negative effect) and favoring a more severe clinical course and a higher frequency of arrhythmia-related cardiac events.7,12
Mutations Related to the Slowly Activating Delayed Rectifier Potassium Current (IKs)
IKs contributes to human atrial and ventricular repolarization, particularly during action potentials of long duration, and plays an important role in determining the rate-dependent shortening of the cardiac action potential. As heart rate increases, IKs increases because channel deactivation is slow and incomplete during the shortened diastole. This allows IKs channels to accumulate in the open state during rapid heart rates and contribute to the faster rate of repolarization.43,44 Importantly, IKs is functionally upregulated when other repolarizing currents (such as IKr) are reduced, potentially serving as a “repolarization reserve” and a safeguard against loss of repolarizing power (see Chap. 2). Mutations in LQT1, LQT5, and LQT11 result in attenuation of IKs and, as a consequence, prolongation of repolarization, action potential duration, and QT interval.7,43 LQT1 is caused by loss-of-function mutations of the KCNQ1 (KvLQT1) gene, which encodes the alpha subunit (Kv7.1) of the inward IKs. More than 170 mutations of this gene have been reported, comprising many Romano-Ward (autosomal dominant) syndromes and accounting for approximately 45% of all genotyped LQT families.12 Of note, mutations involving the transmembrane domain of KCNQ1 result in more severe disease compared with C-terminal mutations.
LQT5 is caused by loss-of-function mutations of the KCNE1 gene, which encodes the beta subunit (MinK) that modulates IKs.7
Homozygous or compound heterozygous loss-of-function mutations of either the KCNQ1 or KCNE1 gene cause the autosomal recessive form of LQTS (the Jervell and Lange-Nielsen syndrome). Patients with KCNQ1 mutations (type 1 Jervell and Lange-Nielsen syndrome) have an almost sixfold greater risk of arrhythmic events, whereas patients with KCNE1 mutations (type 2 Jervell and Lange-Nielsen syndrome) appear to be at lower risk. Although the Jervell and Lange-Nielsen syndrome is the most severe among the major variants of LQTS, the parents of Jervell and Lange-Nielsen syndrome patients are generally less symptomatic than other LQT1 patients, despite the fact that they all are heterozygous for the same gene. This is likely related to the observation that most of the LQT1 genetic variants are missense mutations exerting a dominant-negative effect, whereas most (74%) Jervell and Lange-Nielsen mutations of KCNQ1 are frame-shift/truncating mutations that are unable to cause dominant-negative suppression but are likely to interfere with subunit assembly. Jervell and Lange-Nielsen syndrome accompanies complete loss of IKs in hair cells and endolymph of the inner ear, which results in congenital deafness.6
LQT11 is caused by loss-of-function mutations of the AKAP9 gene, which encodes an A-kinase anchoring protein (Yotiao), shown to be an integral part of the IKs macromolecular complex. The presence of Yotiao is necessary for the physiological response of the IKs to beta-adrenergic stimulation. LQT11 mutations reduce the interaction between Yotiao and the IKs channel (Kv7.1), preventing the functional response of IKs to cyclic adenosine monophosphate (cAMP) and adrenergic stimulation and causing an attenuation of IKs.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree