Chapter 20 Approach to Paroxysmal Supraventricular Tachycardias
Clinical Considerations
A “supraventricular” origin of a tachycardia implies the obligatory involvement of one or more cardiac structures above the bifurcation of the His bundle (HB), including the atrial myocardium, the atrioventricular node (AVN), the proximal HB, the coronary sinus (CS), the pulmonary veins (PVs), the venae cavae, or abnormal atrioventricular (AV) connections other than the HB (i.e., bypass tracts, BTs).1
Epidemiology
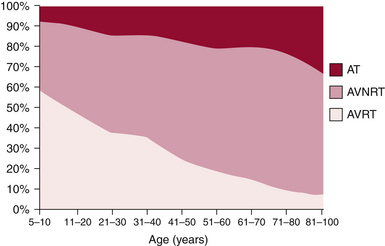
The mechanism of paroxysmal SVT is significantly influenced by both age and gender. In a large cohort of patients with symptomatic paroxysmal SVT referred for ablation, as patients grew older there was a significant and progressive decline in the number of patients presenting with AVRT, which was the predominant mechanism in the first decade, and a striking increase in AVNRT and AT (Fig. 20-1). These trends were similar in both genders, although AVNRT replaced AVRT as the predominant mechanism much earlier in women.2 The early predominance of AVRT is consistent with the congenital nature of the substrate and with the fact that symptom onset occurs earlier in patients with AVRT than AVNRT, most commonly in the first two decades of life. However, a minority of patients have relatively late onset of symptoms associated with AVRT and thus continue to account for a small proportion of ablations in older patients. Men account for a higher proportion of AVRT at all ages.
AVNRT is the predominant mechanism overall in patients undergoing ablation and after the age of 20 years accounts for the largest number of ablations in each age group. AVNRT is unusual in children under 5 years of age, and typically initially manifests in early life, often in the teens. AVRT presents earlier, with an average of more than 10 years separating the time of clinical presentation of AVRT versus AVNRT. There is a striking 2:1 predominance of women in the AVNRT group, which remains without clear physiological or anatomical explanation. Female sex and older age, that is, teens versus early childhood years, favor the diagnosis of AVNRT over AVRT.3
Clinical Presentation
Patients often learn to use certain maneuvers such as carotid sinus massage or the Valsalva maneuver to terminate the arrhythmia, although many require pharmacological treatment to achieve this. In patients without structural heart disease, the physical examination is usually remarkable only for a rapid, regular heart rate. At times, because of the simultaneous contraction of atria and ventricles, cannon A waves can be seen in the jugular venous waveform (described as the “frog” sign). This clinical feature has been reported to distinguish paroxysmal SVT resulting from AVNRT from that caused by orthodromic AVRT. Although the atrial contraction during AVRT will occur against closed AV valves, the longer VA interval results in separate ventricular and then atrial contraction and a relatively lower right atrial (RA) and venous pressure; therefore, the presence of palpations in the neck is experienced less commonly (up to 17%) in patients with AVRT.3 In patients with an AT exhibiting AV block, usually of the Wenckebach type, the ventricular rate is irregular.
Principles of Management
Acute Management
The AVN action potential is calcium channel–dependent, and the non–dihydropyridine calcium channel blockers verapamil and diltiazem are effective for terminating AVN-dependent paroxysmal SVT. The recommended dosage of verapamil is 5 mg intravenously over 2 minutes, followed in 5 to 10 minutes by a second 5- to 7.5-mg dose. The recommended dose of diltiazem is 20 mg intravenously followed, if necessary, by a second dose of 25 to 35 mg. Paroxysmal SVT termination should occur within 5 minutes of the end of the infusion, and over 90% of patients with AVN-dependent paroxysmal SVT respond. As with adenosine, transient arrhythmias, including atrial and ventricular ectopy, AF, and bradycardia, can be seen after paroxysmal SVT termination with calcium channel blockers. Hypotension can occur with calcium channel blockers, particularly if the paroxysmal SVT does not terminate. Adenosine and verapamil have been reported to have a similar high efficacy in terminating paroxysmal SVT, with a rate of success ranging from 59% to 100% for adenosine and from 73% to 98.8% for verapamil, according to the dose and mode of administration. However, data also suggest that the efficacy of adenosine and verapamil is affected by the arrhythmia rate. Increasing SVT rates are significantly associated with higher percentages of sinus rhythm restoration following treatment with adenosine. In contrast, the efficacy of verapamil in restoring sinus rhythm was inversely related to the rate of paroxysmal SVT.4
AVN-dependent paroxysmal SVT can present with a wide QRS complex in patients with fixed or functional aberration, or if a BT is used for anterograde conduction. Most wide complex tachycardias, however, are caused by mechanisms that can worsen after intravenous administration of adenosine and calcium channel blockers. Unless there is strong evidence that a wide QRS tachycardia is AVN-dependent, adenosine, verapamil, and diltiazem should not be used.
Electrocardiographic Features
Assessment of Regularity of the Supraventricular Tachycardia
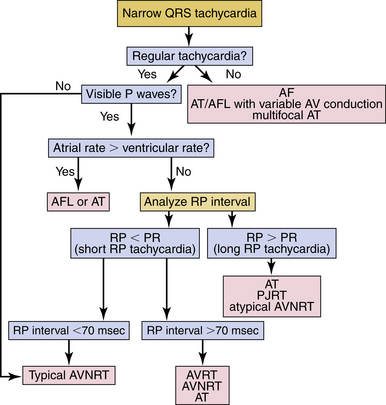
Most SVTs are associated with a regular ventricular rate. If the rhythm is irregular, the ECG should be scrutinized for discrete atrial activity and for any evidence of a pattern to the irregularity (e.g., grouped beating typical of Wenckebach periodicity). If the rhythm is irregularly irregular (i.e., no pattern can be detected), the mechanism of the arrhythmia is either multifocal AT or AF (Fig. 20-2). Multifocal AT is an irregularly irregular atrial rhythm characterized by more than three different P wave morphologies, with the P waves separated by isoelectric intervals and associated with varying P-P, R-R, and PR intervals (see Fig. 11-1). On the other hand, AF is characterized by rapid and irregular atrial fibrillatory activity and, in the presence of normal AVN conduction, by an irregularly irregular ventricular response. P waves cannot be detected in AF, although coarse fibrillatory waves and prominent U waves can sometimes give the appearance of P waves. At times, the fibrillatory activity is so fine as to be undetectable.
Atrial Activity
Identification
If the patient’s rhythm is regular or has a clearly discernible pattern, the ECG should next be assessed for P waves (atrial activity).5 The P waves may be easily discernible; however, frequently, comparison with a normal baseline ECG is needed and can reveal a slight alteration in the QRS, ST segment, or T waves, suggesting the presence of the P wave. If the P waves cannot be clearly identified, carotid sinus massage or the administration of intravenous adenosine may help clarify the diagnosis. These maneuvers may also terminate the SVT.
Carotid Sinus Massage
Carotid sinus massage can result in one of four possible effects: (1) temporary decrease in the atrial rate in patients with sinus tachycardia or automatic AT; (2) slowing of AVN conduction and AVN block, which can unmask atrial electrical activity—that is, reveal P waves or flutter waves in patients with AT or AFL by decreasing the number of QRS complexes that obscure the electrical baseline; (3) with some SVTs that require AVN conduction, especially AVNRT and AVRT, the transient slowing of AVN conduction can terminate the arrhythmia by interrupting the reentry circuit; less commonly, carotid sinus massage can cause some ATs to slow and terminate; or (4) in some cases, no effect is observed.
Termination of the Arrhythmia
Carotid sinus massage or adenosine can terminate the SVT, especially if the rhythm is AVNRT or AVRT. A continuous ECG tracing should be recorded during these maneuvers, because the response can aid in the diagnosis.5 Termination of the tachycardia with a P wave after the last QRS complex is most common in AVRT and typical AVNRT and is rarely seen with AT (see Fig. 18-22), whereas termination of the tachycardia with a QRS complex is more common with AT, atypical AVNRT, and permanent junctional reciprocating tachycardia (PJRT; see Fig. 18-19). If the tachycardia continues despite development of AV block, the rhythm is almost certainly AT or AFL; AVRT is excluded and AVNRT is very unlikely.
Characterization
P Wave Morphology
A P wave morphology identical to sinus P wave suggests sinus tachycardia, inappropriate sinus tachycardia, sinoatrial nodal reentrant tachycardia, or AT arising close to the region of the sinus node. An abnormal P wave morphology can be observed during AVNRT (P wave is concentric; see Fig. 17-3), AVRT (P wave can be eccentric or concentric; see Fig. 18-9), AT (P wave can be eccentric or concentric), and AFL (lack of distinct isoelectric baselines between atrial deflections is suggestive of AFL, but can also be seen occasionally in AT; see Fig. 12-2).5
Characterization of the P/Qrs Relationship
RP/PR Intervals
SVTs are classified as short or long RP interval SVTs (see Fig. 20-2). During short RP SVTs, the ECG will show P waves inscribed within the ST-T wave with an RP interval that is less than half the tachycardia R-R interval. Such SVTs include typical AVNRT (most common), orthodromic AVRT, AT with prolonged AV conduction, and slow-slow AVNRT. A very short RP interval (<70 milliseconds) excludes AVRT.5,6
In typical AVNRT, the P wave is usually not visible because of the simultaneous atrial and ventricular activation. The P wave may distort the initial portion of the QRS (mimicking a q wave in inferior leads) or lie just within the QRS (inapparent) or distort the terminal portion of the QRS (mimicking an s wave in inferior leads or r′ in V1; see Fig. 17-3).
Atrial-Ventricular Relationship
SVTs with an A/V ratio of 1 (i.e., equal number of atrial and ventricular events) include AVNRT, AVRT, and AT. On the other hand, an A/V ratio during the SVT of greater than 1 indicates the presence of AV block and that the ventricles are not required for the SVT circuit, thereby excluding AVRT and suggesting either AT (most common; see Fig. 11-2) or AVNRT (rare; see Fig. 17-4). AV dissociation (i.e., complete AV block) can be observed during AT (most common) or AVNRT (rare).
Electrophysiological Testing
Programmed Electrical Stimulation during Normal Sinus Rhythm
The programmed stimulation protocol should include (1) ventricular burst pacing from the right ventricular (RV) apex (down to pacing CL at which VA block develops); (2) single and double ventricular extrastimuli (VESs, down to the ventricular effective refractory period, ERP) at multiple CLs (600 to 400 milliseconds) from the RV apex; (3) atrial burst pacing from the high right atrium (RA) and coronary sinus (CS; down to the pacing CL at which 2:1 atrial capture occurs); (4) single and double atrial extrastimuli (AESs, down to the atrial ERP) at multiple CLs (600 to 400 milliseconds) from the high RA and CS; and (5) administration of isoproterenol infusion (0.5 to 4 µg/min) as needed to facilitate tachycardia induction.
Atrial Extrastimulation and Atrial Pacing During Normal Sinus Rhythm
Dual Atrioventricular Nodal Physiology
Although the demonstration of dual AVN physiology during programmed atrial stimulation favors AVNRT as the mechanism of SVT, it is not an uncommon finding in patients with other types of SVTs. Furthermore, failure to demonstrate dual AVN physiology does not exclude the possibility of AVNRT, and might be related to similar fast and slow AVN pathway ERPs. Dissociation of refractoriness of the fast and slow AVN pathways can then be necessary (see Chap. 17).
Ventricular Preexcitation
Atrial stimulation can help unmask preexcitation if it is not manifest during NSR because of fast AVN conduction, slow BT conduction, or both. AES and atrial pacing from any atrial site result in slowing of AVN conduction and, consequently, unmask or increase the degree of preexcitation over the AV BT (see Fig. 18-4). Moreover, atrial stimulation close to the AV BT insertion site results in maximal preexcitation and the shortest P-delta interval because of the ability to advance the activation of the AV BT down to its ERP from pacing at this site caused by the lack of intervening atrial tissue, whose conduction time and refractoriness can otherwise limit the ability of the AES to stimulate the BT prematurely (see Fig. 18-14).
Extra Atrial Beats
Atrioventricular Nodal Echo Beats
These beats occur in the presence of anterograde dual AVN physiology (see Fig. 4-23). Such beats require anterograde block of the atrial stimulus in the fast AVN pathway, anterograde conduction down the slow pathway, and then retrograde conduction up the fast pathway. AVN echo beats have several features: they appear reproducibly after a critical AH interval; the atrial activation sequence is consistent with retrograde conduction over the fast pathway, with the earliest atrial activation site in the HB; and the VA interval is very short, but it can be longer if the atrial stimulus causes anterograde concealment (and not just block) in the fast pathway.
Atrioventricular Echo Beats
AV echo beats occur secondary to anterograde conduction of the atrial stimulus over the AVN-HPS and retrograde conduction over an AV BT (concealed or bidirectional BT). If preexcitation is manifest during atrial stimulation, the last atrial impulse inducing the echo beat will demonstrate loss of preexcitation because of anterograde block in the AV BT, and atrial activation sequence and P wave morphology of the echo beat will depend on the location of the BT (see Fig. 3-10). These beats have a relatively short VA interval, but always longer than 70 milliseconds. Moreover, the VA interval of the AV echo beat remains constant, regardless of the varying coupling interval of the AES triggering the echo beat (VA linking). Alternatively, AV echo beats can occur secondary to anterograde conduction of the atrial stimulus over a manifest AV BT and retrograde conduction over an AVN, in which setting the last paced beat is associated with anterograde block in the AVN and fully preexcited QRS complex.
Ventricular Extrastimulation and Ventricular Pacing During Normal Sinus Rhythm
Retrograde Dual Atrioventricular Nodal Physiology
Demonstration of retrograde dual AVN physiology during programmed ventricular stimulation suggests AVNRT (occurring most commonly during atypical AVNRT), but it can also be observed with other SVTs.7 Importantly, failure to demonstrate retrograde dual AVN physiology in patients with AVNRT can be the result of similar fast and slow AVN pathway ERPs, in which setting dissociation of refractoriness of the fast and slow AVN pathways is required (see Chap. 17).
Retrograde Atrial Activation Sequence
VA conduction over the AVN produces a classic concentric atrial activation sequence starting in the anteroseptal or posteroseptal region of the RA because of retrograde conduction over either the fast or the slow AVN pathways, respectively. In the presence of a retrogradely conducting AV BT, atrial activation can result from conduction over the AV BT, over the AVN, or a fusion of both (see Fig. 18-16). An eccentric atrial activation sequence in response to ventricular stimulation suggests the presence of an AV BT mediating VA conduction (see Fig. 18-16). The presence of a concentric retrograde atrial activation sequence, however, does not exclude the presence of a retrogradely conducting BT that could be septal in location or located far from the pacing site, allowing for preferential VA conduction over the AVN.
Extra Ventricular Beats
Bundle Branch Reentrant Beats
During RV stimulation at close coupling intervals, progressive retrograde conduction delay and block occur in the right bundle branch (RB), so that retrograde HB activation occurs via the left bundle branch (LB). At this point, the His potential usually follows the local ventricular electrogram. Further decrease in the coupling interval produces an increase in retrograde HPS conduction delay. When a critical degree of HPS delay (S2-H2) is attained, the impulse can return down the initially blocked RB and result in a QRS of similar morphology to the paced QRS at the RV apex—specifically, it will look like a typical left bundle branch block (LBBB) pattern with left axis deviation because ventricular activation originates from conduction over the RB. The His bundle–ventricular (HV) interval of the bundle branch reentrant (BBR) beat is usually longer than or equal to the HV interval during NSR. Retrograde atrial activation, if present, follows the His potential (see Fig. 4-27).
Atrioventricular Node Echo Beats
These beats are caused by reentry in the AVN in patients with retrograde dual AVN physiology (see Fig. 17-11). The last paced beat conducts retrogradely up the slow AVN pathway and then anterogradely down the fast pathway to produce the echo beat. AVN echoes appear reproducibly after a critical H2-A2 interval (or V2-A2 interval, when the His potential cannot be seen), and manifest as extra beats with a normal anterograde QRS morphology and atrial activity preceding the His potential before the echo beat. This phenomenon can occur at long or short coupling intervals and depends only on the degree of retrograde AVN conduction delay. In most cases, this delay is achieved before the appearance of a retrograde His potential beyond the local ventricular electrogram (i.e., before retrograde block in the RB).
Atrioventricular Echo Beats
These beats occur secondary to retrograde block in the HPS-AVN and VA conduction over an AV BT, followed by anterograde conduction over the AVN, or secondary to retrograde block in the AV BT and VA conduction over the AVN-HPS, followed by anterograde conduction over the AV-BT. In the latter setting, the echo beat is fully preexcited (see Fig. 18-16).
Right Bundle Branch Block during Ventricular Extrastimulation
In the absence of a BT, the AVN can be activated in a retrograde fashion only after retrograde activation of the HB; as a consequence, VA activation will necessarily be delayed with retrograde RBBB, and the increase in the VA interval will be at least as much as the increase in the VH interval. In contrast, when retrograde conduction is via a BT, there will be no expected increase in the VA interval when retrograde RBBB is induced. Thus, the increase in the VA interval is minimal and always less than the increase in the VH interval.8
Induction of Tachycardia
Initiation by Atrial Extrastimulation or Atrial Pacing
Inducibility
All types of paroxysmal SVTs can be inducible with atrial stimulation (except automatic AT). SVT initiation that is reproducibly dependent on a critical AH interval is classic for typical AVNRT (see Fig. 17-8). Atypical AVNRT is usually initiated with modest prolongation of the AH interval along the fast pathway with anterograde block in the slow pathway, followed by retrograde slow conduction over the slow pathway. Therefore, a critical AH interval delay is not obvious (see Fig. 17-10). AT initiation also can be associated with AV delay, but that is not a prerequisite for initiation. Orthodromic AVRT usually requires some AV delay for initiation; however, the delay can occur anywhere along the AVN-HPS axis. In patients with baseline manifest preexcitation, initiation of orthodromic AVRT is usually associated with anterograde block in the AV BT and loss of preexcitation following the initiating atrial stimulus, which would then allow that BT to conduct retrogradely during the SVT.9 Initiation may require catecholamines (isoproterenol) with any type of SVT, and this observation does not help for differential diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree