A 70-old-male patient was admitted to the emergency room with a history of falling at home and inability to walk. An intertrochanteric fracture of the right femur was confirmed by X ray studies and examination. Intramedullary nailing for unstable fracture was planned. Prompt prophylaxis for thromboembolism was initiated with 0.4 IU of enoxoparine while anesthesiologic stabilization of internal diseases of the patient continued. A closed nailing of the fracture was performed with epidural anesthesia the following day. On the postoperative first day, a hemorrhagic discharge and staining of the dressings was revealed. There was an inequality of radius of right thigh and extensile subcutaneous hemorrhagic ecchymosis was revealed. Medical prophylaxis was stopped and prophylaxis was continued with mechanical prophylactic measures and encouragement of early mobilization.
The risk analysis for thrombophylaxis was assessed with the Padua Score. The age of the patient, obesity, the planned orthopedic operation, and prolonged immobilization pose a high risk of thromboembolism to the patient [1]. A dose of 0.4 IU of enoxoparine is appropriate for a patient weighing 70 kg. Although prophylaxis lowers the risk of thrombophylaxis, it can cause exaggerated hemorrhagia at the postoperative surgical site [2]. In case of such an event, discontinuation of low-molecular-weight heparins until hemostasis is obtained is advised. Although low-molecular-weight heparins lower the risk for thromboembolic events, they can cause some secondary morbidities with the increase of hemorrhagic events.
Introduction
Venous thromboembolism (VTE) is the general name of all pathological thrombosis in the venous circulation. The three main components of VTE are deep vein thrombosis (DVT), chronic venous disease, and pulmonary embolism. It occurs most often in lower-extremity deep veins and rarely in upper-extremity, pelvic, and other veins. The most important component of VTE that threatens life is pulmonary embolism (PE). Two million DVT cases and 600,000 PE cases are seen in the United States annually. In addition, about 200,000 people die each year in the United States from PE. VTE is a disease with more than one cause. and multiple risk factors may coexist simultaneously in patients. The more risk factors a patient has, the higher the risk of developing VTE (Tables 40.1 and 40.2). Three basic pathogenetic mechanisms that facilitate the development of VTE are described by Virchow. Virchow’s triad includes slowing of blood flow (stasis), damage to the blood vessel wall (primarily endothelial damage and dysfunction), and hypercoagulability [3]. These pathogenetic mechanisms are still accepted and genetic changes (polymorphisms/mutations) were added to these mechanisms with today’s technology (Table 40.3).
Table 40.1
Fatal DVT and PE ratios determined by venography
Without prevention (prophylaxis) | DVT | Fatal PE |
|---|---|---|
Total hip prosthesis | 42–57 % (Proximal DVT: 18–36 %)a | 1–2 % |
Total knee arthroplasty | 41–85 % (Proximal DVT: 5–22 %)a | 0.1–1.7 % |
Open menisectomy | 20 % | Unknown |
Femur fracture | 60 % | 3.5 % |
Spinal trauma + paralysis | 100 % | 1 % |
Multivariate trauma | 35–58 % | Unknown |
Pelvis fracture | 20–60 % | Unknown |
Table 40.2
PE ratios
With prevention (prophylaxis) | PE (%) | Fatal PE (%) |
|---|---|---|
Total hip prosthesis | 0.9–28 | 1–2 |
Total knee arthroplasty | 1.5–10 | 0.1–1.7 |
Total hip prosthesis + warfarin prophylaxis | <0.1 |
Table 40.3
The influential factors in the formation of Virchow’s triad
Venous stasis | Injury on blood vessel wall | Hypercoagulability |
|---|---|---|
Long-term bed rest, long travel, inactivity that depends on the surgery operation Tumors, obesity, pregnancy-related venous obstruction Cardiomyopathy, congestive heart failure, and myocardial infarction dependent left ventricular failure Atrial fibrillation | Vein injury/traumata Catheter insertion Deep vein thrombosis formation (varicose vein formation + valve damage) Artificial heart valve Acute myocardial infarction Surgical intervention Bone fractures Cardiovascular disease Tumor invasion Ambustion | Acquired thrombophilias History of deep vein thrombosis Surgical procedures Antiphospholipid antibody syndrome Others Hereditary thrombophilias Frequently seen Activated protein C Resistance Factor V Leiden mutation Prothrombin gene (G20210A) mutation Protein C/S deficiencies Lack of antithrombin Rarely seen Family history |
Coagulation Mechanism
Coagulation is the formation of fibrin by the interaction of more than a dozen proteins. After extrinsic and intrinsic pathways independently start, the coagulation engages in a common way. The damage of the subendothelial area of a vein activates the intrinsic pathway by activation of factor VII, which is measured with partial thromboplastin time (PTT). The extrinsic pathway is activated by thromboplastin, which is released into circulation after the cellular damage and measured with prothrombin time (PT). The common path begins with the activation of Factor X. Activated Factor X generates thrombin from prothrombin. Thrombin works agonist with thrombocytes. Thrombin converts fibrinogen to fibrin and activates factor XIII, which stabilizes the fibrin [4] (Fig. 40.1).
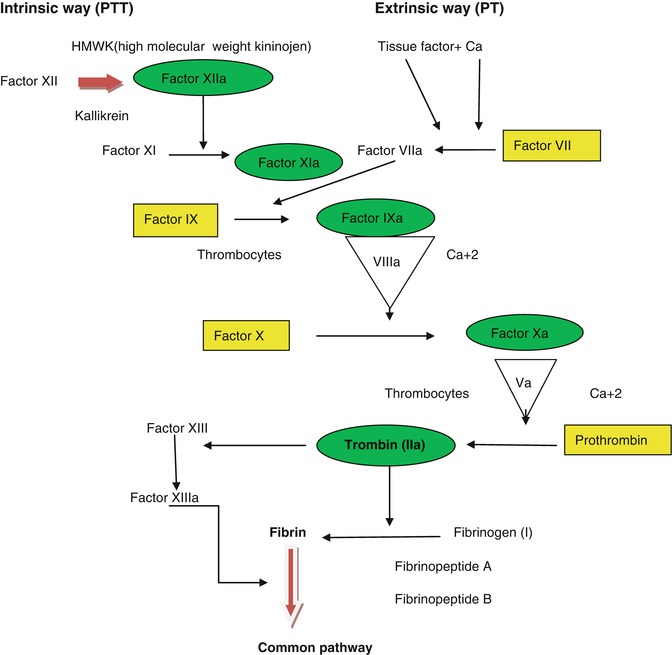
Fig. 40.1
Intrinsic and extrinsic coagulation pathways. Rectangular shapes vitamin K-dependent factors – sensitive to warfarin, Oval shapes inhibited factors in heparin side – antithrombin III
Fibrinolytic System
The fibrinolytic system prevents clotting and maintains vessel patency. There are natural mechanisms functioning opposite of coagulation. There are three systems; antithrombin, proteins C and S, and plasminogen-plasmin. Antithrombin binds thrombin, proteins C and S inhibit factors Va and VIIIa, and plasmin destroys fibrin [5]. If the coagulation and fibrinolytic systems becomes unbalanced, venous thrombosis occurs because of stasis, endothelial damage, and hypercoagulability, known as Virchow’s triad.
Venous Thromboembolic Disease
Epidemiology and Risk Factors
After major orthopedic surgeries, the prevalence of DVT is between 30 % and 50 %. When VTE prophylaxis is not provided after hip fracture, the prevalence of fatal pulmonary embolism is 7 %. Most sources of PE are proximal veins. Symptomatic PE ratios after electively applied total hip arthoplasty (THA) and total knee arthoplasty (TKA) without prophylaxis are 20 % and 8 %, respectively. DVT was seen more frequently in venographs taken after TKP, but symptomatic PE is seen less than with THA than TKP. Risk factors include age, gender, race, history of DVT, varicose veins, cancer, oral contraceptives, heart failure, obesity, smoking, stroke, immobility, tissue injury, trauma, pregnancy, nephrotic syndrome, and surgical intervention. For example, antithrombin III level drops due to blood loss during THA. The incidence of VTE risk is higher when the femur is cavitated in THA and the femoral side of the prosthesis (especially in cemented prosthesis) is applied.
Age
Advancing age increases the risk of VTE, and age is a strong risk factor for VTE. VTE risk is 0.01 % per year for people under age 40; for older people, the risk increases by 1 % each year. Although the exact cause is unknown, an increase in VTE is seen because of a combination of factors, including decreased mobility, the development of additional diseases, thrombosis, and an increase in coagulability with age [6].
Gender
Although men and women are equally at risk for VTE, it is seen more among young women because of their use of oral contraceptives (OC). VTE is two to four times higher among patients who use OC compared with those who do not.
Race
VTE is seen 25 % more often among Caucasians and African Americans. It is 70 % less common among American Indians, Pacific Islanders, and Hispanics.
Season
According to some meta-analyses, fatal PE and VTE are 43 % more likely during the winter. A decrease in physical activity may be the cause for this.
Obesity
Although the reason remains uncertain, coagulation and inflammation are increased in obese people [7]. The risk of VTE is increased 2 to 3 times if BMI is over 30 kg/m2.
Hormone Therapy
OC users are at a higher risk of VTE, with VTE seen in approximately 85 of 100,000 persons.
Inpatients
In hospitalized patients, venous thrombosis is higher due to immobilization, cancer, and surgery. VTE occurs 100 times more often than in the general population. Fifty percent of hospitalized patients are at risk of VTE, and VTE can occur even 3 months after discharge.
Surgery
Immobility due to surgery and general anesthesia creates the Virchow triad and increases the risk of VTE. Long immobilization in the perioperative period reduces the effects of massage in the calf muscles and causes venous stasis. General anesthesia causes endothelial damage by reducing venous tone. Orthopedic surgery and trauma cause multiple prothrombotic events. After THA, TKA, and hip fracture surgery, asymptomatic DVT is commonly seen [8].
Cancer
VTE is seen among approximately 1 in 200 of cancer patients and if 4.1 times more frequent than in the general population. Cancer cells increase the risk of VTE by activating thrombocytes and coagulation pathways [9].
Thrombophilia
Severe thrombophilia occurs due to antithrombin and protein C and S deficiency. It is not common, but it causes strong thrombosis. Light thrombophilia causes thrombosis by increasing procoagulant factors if there is a deficiency of factor V Leiden and PG20210A [10].
Travel
After traveling by plane, car, train, or bus for more than 4 h, the risk of VTE is increased by nearly 3 times for a few weeks.
VTE Diagnosis
Clinically, VTE and PE have no specific diagnostic symptoms. Pain, swelling, and pain in the calf of the leg by forcing the foot to dorsiflexion (Homans sign) are the symptoms. The examinations used in diagnosis are chest radiography, electrocardiography, arterial blood gases (evaluation of oxygen), ventilation-perfusion scintigraphy, high-resolution spiral chest computed tomography, pulmonary angiography, and Doppler ultrasonography [11]. D-dimer is an additional examination. High D-dimer is an indication of fibrin degradation. Low D-dimer values indicate a low risk of VTE (high negative predictive value) [12]. Venography is the gold standard, but there are undesirable effects, such as its being expensive and invasive and the need for radiation exposure.
Prophylaxis
High risk of thromboembolic events causing morbidity and mortality after major orthopedic surgery and the difficulty of diagnosis usually warrants prophylaxis. There are mechanical and pharmacologic prophylaxis modalities available. The duration of prophylaxis is controversial. There is no consensus, but most of the literature includes the guidelines for prevention of VTE after surgery. In prophylaxis, some studies that are intended to prevent PE are needed, which would be possible with a single-center prospective study involving 50,000 patients.
Mechanical Approaches
A sequential pneumatic pressure-applying apparatus (IPC) prevents the development of stasis by increasing venous current and stimulates the fibrinolytic system so there is no risk of bleeding. Poor patient compliance and misuse are common. Good results have been reported after THA.
Plantar pressure applying devices provide deep vein pulsatile flow by applying pressure to the deep vein plexus in the foot (an effect like walking). The patient should not use this while alone.
Graduated pressurized stockings prevent venous stasis by applying different pressures to the lower and upper levels of the lower extremity. These socks are recommended in addition to medical prophylaxis [13].
Inferior Vena Cava Filters (IVCF)
Indications
1.
If the use of anticoagulants is contraindicated (head and spinal cord injury)
2.
If hemorrhage develops secondary to anticoagulants
3.
If VTE develops despite anticoagulant therapy
4.
If clot continues to grow and duplicate
5.
If the patient has a low cardiopulmonary reserve, prophylactic IVCF is implemented for the patient due to of the high risk of morbidity and mortality. This is expensive and morbidity is high [14].
Pharmacological Prophylaxis
Heparin
Heparin is an indirect thrombin inhibitor. There are different molecular weights (approximately 15,000 Da). The pentasaccharide unit is extended so that, by binding to antithrombin, it also binds factors VII, IX, X, and XL and thrombin. Heparin does not pass the placenta and is the only anticoagulant that can be used safely during pregnancy. Contraindications include hemorrhagic diathesis, malignant hypertension, cerebral hemorrhage, and peptic ulcers. Although dose-adjusted heparin is effective, the possibility of bleeding complications and need for close monitoring are disadvantages. Heparin can cause osteopenia or osteoporosis. Thrombocytopenia and thrombosis, which are formed by development of antibodies against platelets, are seen at the same time. If thrombocytes are reduced by 50 %, it is considered heparin-induced thrombocytopenia (HIT). Hypersensitivity, skin necrosis, alopecia, mild transaminase elevation, and hypoaldosteronism are seen. In cases of HIT, heparin is interrupted and the thrombin inhibitors argatroban and lepirudin are given. Protamine sulfate is heparin’s antidote [15, 16].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








