Chapter 15 Upper limb orthoses for the person with spinal cord injury
Pathophysiology
The cervical spinal cord is encased within seven cervical vertebrae. At the first and second levels, the diameter of the spinal cord is small in relation to the size of the spinal canal. The cord occupies only one third of the canal at C1–2 but occupies half of the canal at C7.45 Because range of motion (ROM) is greatest at the C5, C6, and C7 vertebrae and as a result of the relationship between canal size and cord size in the lower cervical region, the majority of injuries occur at those levels.
Traumatic SCI generally results in disruption of the spinal cord architecture, followed by a complex pattern of pathophysiologic processes that exacerbate the injury.65 Although they may be classified as functionally complete, the majority of traumatic injuries do not result in complete disruption of the cord. Residual injury often is characterized by a peripheral rim of intact tissue with a central cystic cavity.16,55,65 In a so-called “clinically complete” injury, peripherally preserved pathways can result in nonfunctioning residual axons secondary to loss of myelin locally, or they may be involved in the degree of spasticity exhibited. In incomplete SCI, in the condition called central cord syndrome, the rim of damage initially expands outward, but as the degree of injury resorbs back more centrally, the function effected by the periphery becomes preserved. Central cord syndrome typically is characterized by preserved bowel, bladder, and lower extremity function but dramatic loss of upper extremity function. The anatomical reason for this finding is the layering of the fibers in the lateral cortical spinal tract (primary motor tract). These fibers are layered such that the more distal functioning tracts are more peripheral, whereas the fibers for the upper extremity are more central in the tract and therefore more susceptible to damage that occurs from central to peripheral.
In the cervical spine, a unique set of injuries to the white matter and the gray matter results in a combination of central and peripheral injuries. Damage to the white matter affects motor control and sensory input from the periphery to the brain below the level of injury. Almost always at and surrounding the level of injury, evidence is seen of lower motor neuron (LMN) injury resulting in flaccid paralysis at the level of injury.90,118 Muscles with LMN injury are at greater risk for contractures22 as a result of shortening and scarring. In addition, muscles with LMN injury are not amenable to functional electrical stimulation (FES). Coulet et al.22 have detailed the implications of SCI pathophysiology on upper extremity rehabilitation.
SCI most frequently occurs in young adults between the ages of 16 and 30 years,37 with a recent increasing trend in injuries sustained by older persons.100 In the United States, motor vehicle crashes and violence are the primary causes of SCI.26 Approximately 3% to 5% of SCIs that occur each year in North America are sustained by individuals younger than 15 years and about 20% in those younger than 20 years.39,41,59,100 Although boys more commonly sustain SCI than girls during childhood or adolescence, the frequency of SCI in boys and girls younger than 5 years is comparable.39,41,112,132,133 The level and category of neurological injury vary as a function of age, with younger children more likely having paraplegia or complete injuries.132,133 The management, complications, and outcomes of adult and pediatric SCI have been extensively addressed in four notable comprehensive publications.9,50,61,127
Historical perspectives
Although high mortality rates after cervical SCI once were commonplace, today survival and discharge to the community are typical.27 Although complications as a result of SCI still occur,19 they are becoming less associated with medical and physical comorbidities and increasingly are related to challenges associated with resuming meaningful roles and productive work. This is particularly true for individuals with adult-onset tetraplegia who develop physical and select medical problems more frequently than their peers with paraplegia121,122 but also realize greater challenges in dating, marriage, and productive employment.135 Similarly, individuals with childhood-onset tetraplegia are underemployed, date less frequently than typically developing youth and young adults,4 and, once they become adults, are more likely to be overeducated and underemployed.
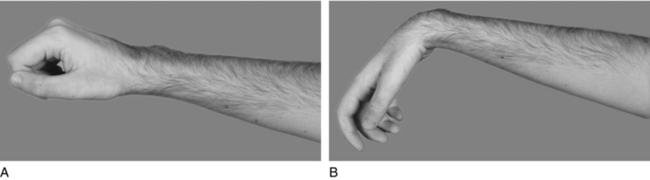
Despite the dramatic progress in medicine, rehabilitation, and societal modifications, the upper limb remains obliged to assume new and demanding roles after SCI. After the introduction of antibiotics, high mortality rates among persons with tetraplegia declined, and early rehabilitation techniques focused on purposeful tightening of the finger flexor muscles to develop force between the thumb and index/middle fingers (Fig. 15-1).45,139,141 Typically referred to as “natural tenodesis,” “tenodesis action,” “passive tenodesis,” and “tenodesis hand,”52,83,113 this pinch, which provides sufficient force to acquire light objects,52 formed the basis for the wrist-driven flexor hinge splint11 and the early techniques for surgical augmentation of the C6 hand.17 Although the tenodesis hand remains a mainstay of rehabilitation in many SCI centers,45,52,122,127 today’s requirements for the upper limb of persons with SCI typically exceed what can be provided by the tenodesis hand. As such, new treatment paradigms have emerged over the last few decades. The evolution of surgical reconstruction and technology has resulted in upper extremity interventions that offer people with tetraplegia not only the long-standing goal of independence but also more contemporary goals associated with spontaneity, ease of activity, performance, and improved cosmesis.31,34,45,58,69,73,93,95,101–103
Surgical reconstruction of the upper limb in tetraplegia: Historical perspective
Early reports of surgical reconstruction of the hand in tetraplegia describe using techniques similar to those used in polio,17,123 with varying degrees of success and little acceptance among rehabilitation phyisicans.45 Erik Moberg is recognized as the father of upper extremity tendon transfers in SCI and, like other experts, pioneered the early reconstructive work. Despite positive outcomes, hand surgery continued to have little if any role in the general rehabilitation of persons with tetraplegia. However, in the last two decades of the 20th century, an international effort to build consensus on treatment techniques and share outcomes emerged.44,73,81,82 As a direct result of this effort, surgical reconstruction of the upper extremity in select SCI centers worldwide has restored elbow extension,91,94–96 wrist extension,33,43 forearm rotation,38 and hand grasp and release.32,33,35,47,66
Elbow extension has commonly been restored by transferring the posterior and part or all of the middle deltoid to the triceps.44,82,91,94,95 However, evidence now suggests that the biceps-to-triceps transfer may provide superior outcomes.91 Although the traditional goal of a tendon transfer for elbow extension has been to restore control of the arm and hand,94 current tendon transfer planning considers restoration of power because of the promise of neurological recovery through regeneration and restorative therapies. For this reason, biceps transfer is being investigated as a preferred alternative to the traditional approach using the deltoid muscle.
Wrist extension provides tenodesis grasp in the absence of voluntary muscles. When combined with soft tissue reconstruction, transfer of the brachioradialis (BR) to the extensor carpi radialis brevis restores passive prehension to persons with a C5 SCI level of injury and has led to their ability to complete activities of daily living (ADLs).33,44,46,82,94,95 Tendon transfers also have been performed to restore active finger and thumb flexion for pinch,47,66,88 grasp,32,35,88 and hand opening.32,43 In combination, these tendon transfers enable persons with C6 and C7 SCI to acquire and hold objects that vary in size and weight using either palmar (gross) grasp or lateral (key) pinch. Tendon transfers for thumb and finger extension make release of objects possible without reliance on gravity-assisted wrist flexion. Restoration of active grasp and release eliminates the need for adaptive equipment for eating, brushing teeth, catheterizing, and other ADLs. When provided bilaterally, it restores bilateral abilities and improves the patient’s ability to negotiate ramps, curbs, and uneven terrain while in the wheelchair by providing stronger grip of the tire rims. Tendon transfers also play an important role for ambulatory persons with incomplete tetraplegia who require restored function to maintain grasp of a walker or a crutch.
Neuroprosthesis for the upper limb in tetraplegia: Historical perspective
In addition to the contributions by Erik Moberg, the pioneering work in the area of FES by Hunter Peckham and Michael Keith represents one of the most influential efforts in the area of upper limb rehabilitation in tetraplegia. In cases of SCI, FES is the coordinated stimulation of two or more muscles to achieve a functional outcome.85 The distinguishing feature between FES and therapeutic electrical stimulation (TES) is twofold. First, the primary purpose of FES is to enable an individual to perform an activity. In contrast, the primary purpose of TES is to provide a pattern of stimulation for therapeutic purposes such as muscle strengthening and ROM. The second primary distinction between FES and TES is the control source. The FES user has voluntary control of the stimulation parameters and variables via a switch or joint sensor and/or programming.53 TES is preprogrammed and not under the user’s volitional control.
Neuroprosthesis is a term coined to describe bracing of the limb by means of FES. The early work of Peckham and Keith using percutaneous electrical stimulation systems restored palmar and lateral grasp and release to persons with midcervical impairments106,107 and demonstrated restored capabilities in ADL.25,93,101,117,118,139 Successful outcomes of percutaneous work led to the development of an implantable neuroprosthesis104,105,116 marketed under the name Freehand System.
The NeuroControl Freehand System, an eight-channel neuroprosthesis consisting of implanted and external components (Fig. 15-2) with capability to restore grasp and release function (Fig. 15-3), gained market approval by the United States Food and Drug Administration (FDA) after an international clinical trial demonstrated significance increases with FES in pinch and grasp forces, improved independence of every subject while using FES, and high satisfaction and preference for FES among users.104 Subanalyses of the clinical trials data showed positive outcomes of the Freehand System on pediatric, adolescent, and adult performance of and satisfaction with ADLs24,60,87,124,126 Because of the strong evidence supporting FES as an efficacious treatment and as a direct response to consumers’ requests for the device early in recovery, the Freehand System also has been used successfully for restoring function during initial SCI rehabilitation.86 Yet, despite the evidence outcomes and other23 support of neuroprostheses, marketing of the device was stopped in 2001.57 Next-generation implantable neuroprostheses systems are under development57,101 and are anticipated to reemerge in the 21st century as an intervention and treatment of choice for restoring upper extremity in persons with tetraplegia.
Some FES systems have used surface electrodes. The bionic glove, which was developed in 1989 by researchers at the University of Alberta, underwent multiple design modifications for improvement in functionality109 for persons with midcervical tetraplegia. The glove requires the application of surface electrodes and donning of the glove that, when secure, holds the electrodes in place. Among nine users, only one reported difficulty in donning. Voluntary wrist extension provides the control source for stimulated grasp. Preliminary results with the bionic glove were positive, as evidenced by 24% and 49% improvement in the Functional Independence Measure and Quadriplegic Index of Function, respectively.109 Plans for clinical deployment of the bionic glove are unclear.
The NESS H200 (Bioness Inc., Santa Clara, CA), formerly known as the Handmaster, consists of an arm–hand orthosis and a control box. Five surface electrodes contained within the orthosis deliver stimulation to the long extensor and flexor tendons of the fingers and thumb and to intrinsic thenar muscles.120 Preliminary reports of 10 consecutive patients showed that six reestablished grasp patterns that enabled manipulation of objects that was not achievable without the device.120 Although no prospective studies with large number of persons using the system have been performed, Alon and McBride2 demonstrated positive outcomes of the NESS H200 in a series of patients with midcervical SCI.
Clinical directions of FES for the 21st century are well described by Peckham and Gorman101 and include systems that will address multisystem functions, spontaneous and natural control mechanisms, and modularity, allowing for upgrades as technology advances. Hybrid systems likely will emerge as clinically relevant treatment paradigms emerge for upper limb neuroprostheses that combine FES, orthoses, and reconstructive surgery.
Orthotic intervention: Historical perspective
Until the introduction of neuroprostheses, the principles of upper extremity functional orthotic intervention for persons with tetraplegia remained relatively unchanged. Early work with patients with poliomyelitis49 paved the way for orthotic treatment guidelines in tetraplegia,28,48,64,95,97,99,140 many of which remain pertinent today.
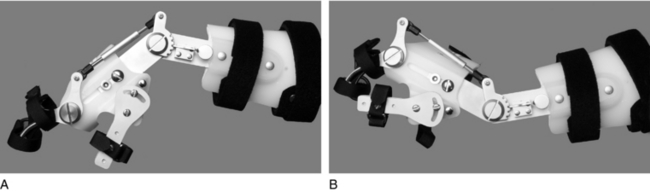
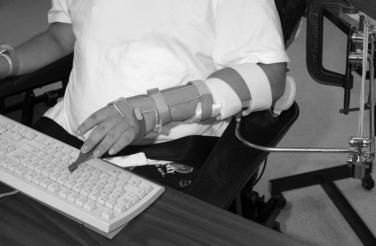
Whether powered by external sources3,6,75,128 or voluntary wrist extension,5,54,62,75,80 the wrist-flexor hinged orthosis (Fig. 15-4) has been and continues to be a mainstay of orthotic prescription in tetraplegia rehabilitation. The first report on the wrist-driven flexor hinged orthosis described a static position of the stabilized thumb and partially flexed index and middle fingers that allowed for pinch with active wrist extension and opening with gravity-assisted wrist flexion.97 If finger motion was preserved, a finger-driven orthosis was prescribed.45 This variation has not remained a popular intervention most likely because of the splint’s interference with sensory input to a sensate hand and poor cosmesis.
Nickle’s wrist-drive flexor hinged orthosis, which was based on biomechanical principles of the hand13 and commonly referred to as the “Rancho Splint” or “wrist-driven flexor hinge splint,” was prescribed during stages of rehabilitation when training in multiple activities was being conducted.56,98 For persons without wrist extension, cable-driven and gas-powered sources were used to activate the pinch.6,29 Both sources of power were minimally accepted by the users because of the effort required, donning issues, and poor comfort; as a result, both have been phased out.45 However, despite rejection of the externally powered flexor hinge splint by persons with higher cervical injuries, a variety of wrist-driven orthoses remain on the market for those with midcervical injuries. These wrist-driven orthoses are considered the standard of care for persons with midcervical tetraplegia for restoring simple ADL function (Fig. 15-5).5,21,28,48,54,74,84
Mobile arm supports (MAS) and suspension slings are designed to aid persons with high tetraplegia (C4 and above) with hand-to-mouth and tabletop activities. Although the terms are used interchangeably, as shown in Figure 15-6, MAS are mechanical devices that support the arm and forearm and assist with shoulder and elbow movement through a linkage of ball-bearing joints,69,70,75,84,115,128 whereas suspension slings are kinetic devices that support the upper extremity.84,127 The suspension feeder was first introduced for persons with polio49 to assist with hand-to-mouth activities. Forearms rested in a trough and the arms were suspended by cables or springs from an overhead frame attached to the wheelchair. Although effective for some persons with weak residual proximal control, the suspension feeder eventually was rejected by the population with SCI because the bulkiness of the device made wheelchair dimensions exceed accessible values, and portability from one environment to another was cumbersome.
Development of next-generation feeders focused on smaller devices positioned beneath the user’s arm or mounted to the table. For example, the StandFeeder was a table-mounted unit with the pivot point located under the forearm,69 and the C-Clamp Feeder was mounted to the tabletop and incorporated a forearm trough and the flexor hinged orthosis.8
Early work on the overhead suspension system and feeders paved the way for development of the ball-bearing feeder,118 linkage feeder, and balanced forearm orthosis,69 all of which are collectively referred to as MAS. Two of the first MAS introduced to persons with tetraplegia were developed by engineers at Rancho Los Amigos and Texas Institute for Rehabilitation and Research (TIRR).69 The Rancho MAS, also called the Golden Arm, was successfully used by persons with polio who, despite no muscle activity, had full sensation in their limbs; the Golden Arm was rejected by those with SCI because of lack of sensory feedback while using the device.69
Engen’s early work at TIRR provided important contributions to the philosophy of upper limb orthotic management that remain pertinent for SCI care today. In addition to engineering and design elements of the orthoses,29 Engen believed that users must have easy control of the device and, as a result, custom made each MAS to meet the unique needs of individual patients. This custom approach to MAS restored three major arm and hand functions69 and provided ease of function to the recipients. However, despite sound concepts and excellence in design, Engen’s custom-made MAS was ill suited for commercial production and therefore eventually failed in successful clinical deployment.
The A.I. DuPont Hospital for Children has contributed to the evolution of MAS for persons with SCI.42 Most recently they have focused on development of a power-assisted arm orthosis, the Wilmington Robotic Exoskeleton (WREX).110,111 The WREX, which is mounted to the wheelchair, uses linear elastic elements to counter gravity and power assistance to shift the arm in response to the user. Preliminary trials of the WREX with persons having muscular dystrophy showed more than 50% reduction in the time to complete items on the Jebsen Test of Hand Function.111 Development of free-standing arm units, such as the Action Arm (Flaghouse, Hasbrouck Heights, NJ) and the Zoncoarm (Technology for Education, Inner Grove Heights, MN), are being developed and illustrate the continued need for effective orthoses for high tetraplegia.
Current issues
Despite the evolution of upper extremity treatment for persons with tetraplegia, significant clinical challenges in SCI rehabilitation for persons with tetraplegia remain. No method effectively restores functional use of the upper limb in patients with high tetraplegia. Rehabilitation engineers in research laboratories continue to develop techniques, but pathways for clinical deployment are ill defined.69 Tabletop feeding assists remain in use because of the limitations in restoring effortless and efficient upper extremity function to patients, particularly those with the most severe injuries.
Strong evidence supports use of neuroprostheses in patients with midcervical injuries, but knowledge of device availability and perceived complexity remain barriers to widespread use. Also, the only FDA-approved implantable FES system for upper limb function failed in the market because of the high cost and relatively small population with tetraplegia, and FES systems using surface electrodes have not gained acceptance by the rehabilitation community. Yet, despite these challenges, evidence in support of neuroprostheses is building, and promising clinical work continues. This work likely will impact standard of care within a few years.101
Evidence from clinical series and retrospective reviews in support of tendon transfers is strong, yet few centers offer a comprehensive program presumably because of the disconnect between the hand surgeon and rehabilitation physician.66 Although the functional gains are enormous, the postoperative course associated with tendon transfers requires a temporary setback in independence, and, for some individuals who have been living with SCI, this sacrifice cannot be made because of financial, family, and work obligations. In recognition of this challenge, contemporary clinicians suggest that tendon transfers be considered during the immediate rehabilitation period when neurological stability is reached.
Orthotic intervention continues to be a mainstay in tetraplegia rehabilitation. Despite proposed recommendations,21 acceptance and implementation of clinical practice guidelines are lacking, and most therapists have inadequate education on how to train people with SCI on the use of orthoses and neuroprostheses for activities.69,101
Other relevant issues should be considered. In the era of accountability and evidence-based practice, no level I studies support upper limb interventions in SCI. Although a plethora of literature on orthotic, reconstructive, and FES procedures is available, few prospective studies have used rigorous research methodology. A major barrier to outcomes research is the lack of a universally accepted outcome measure for upper limb function in SCI69 and the relatively few number of persons with tetraplegia who are able or willing to participate in clinical outcomes trials.
There are broader issues as well. The 21st century brings an increase in the frequency of incomplete injuries and promising regeneration clinical trials that hold promise for some degree of motor and sensory recovery. Persons with incomplete tetraplegia who have more atypical pain and spasticity may be less desirable candidates for hand surgery, FES, or orthoses. Conversely, some persons with incomplete tetraplegia may be fully ambulatory using assistive devices, thus placing much more demand on upper limb function, whether it be preserved or restored. Persons with complete and incomplete SCI are living longer, and new complications associated with aging, such as pain and degenerative changes of the upper limb, are becoming more prevalent.20