Chapter 2 Understanding Muscle Contraction*
After studying this chapter, the reader will be able to do the following:
MUSCULAR ASPECTS OF MOVEMENT
Overview of Muscle Tissue
Functions of Skeletal Muscle
Although locomotion is the primary function of skeletal muscle tissue, the muscular system also performs other important roles. In addition to locomotion and manipulation, skeletal muscles maintain body posture, assist in venous return of blood to the heart, and play an important role in thermogenesis (heat generation). Heat is a by-product of cellular respiration; and because muscles use a great deal of energy for movement, they also generate a great deal of heat. Additionally, muscles act as energy transducers by converting biochemical energy from ingested food into mechanical and thermal energy. Skeletal muscles also protect internal organs and make up most of the protein in the body. Because muscles represent such a large amount of protein, they constitute a potential but rarely used form of stored energy. The use of protein as an energy substrate is discussed in Chapter 3.
Macroscopic Structure of Skeletal Muscles
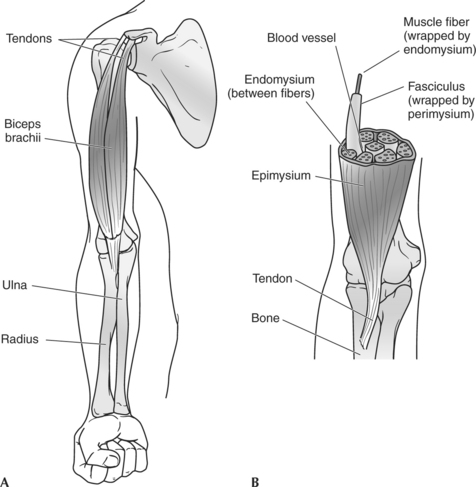
The human body contains more than 400 skeletal muscles, and they account for 40% to 45% of the adult male body weight and 23% to 25% of the adult female body weight.1,2 These muscles function together in a remarkable way to provide smooth, integrated movement for a wide variety of activities, many of which require little conscious thought. Muscle action also provides the basis for sport and fitness activities, and muscle definition itself, as seen in Figure 2-1, has become the goal of the sport of bodybuilding. To understand how muscles function in bodybuilding poses, or in any other activity, it is necessary to look beneath the skin.
Organization and Connective Tissue
Skeletal muscles are organized in a systematic fashion, as depicted in Figure 2-2. Some of this organization is apparent to the naked eye, but other aspects are apparent only when muscle fibers are viewed through a simple or an electron microscope.
Architectural Organizations
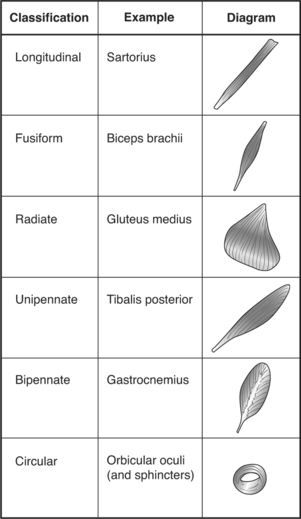
Different arrangements of fasciculi within a muscle account for the different shapes that a muscle may take. Muscles can be described as longitudinal, fusiform, radiate, unipennate, bipennate, or circular, as shown in Figure 2-3. The shape of the muscle determines its range of motion and influences its power production. The longer and more parallel muscle fibers, as found in longitudinal muscles, allow for greater muscle shortening. Bipennate and multipennate muscles, by contrast, shorten little but are more powerful.
Microscopic Structure of a Muscle Fiber
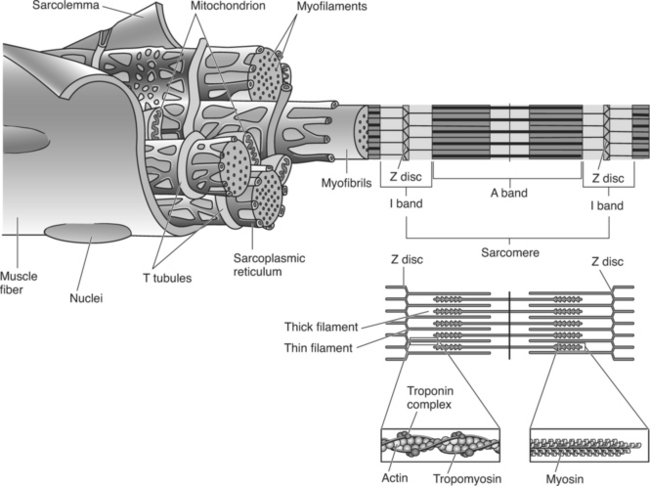
Individual muscle fibers are composed primarily of smaller units called myofibrils, which are in turn made up of myofilaments. This organization of skeletal muscle is shown in Figure 2-4.
Muscle fibers, also called muscle cells, are long, cylinder-shaped cells ranging from 10 to 100 μm in diameter and 1 to 400 mm in length.2–5 The major structures of a muscle fiber and their functions are summarized in Table 2-1.
Table 2-1 Summary of Major Components of a Skeletal Muscle Cell
| Cell Part | Description | Function |
|---|---|---|
| Nucleus | Multinucleated | Is the control center for the cell |
| Sarcolemma | Polarized cell membrane | Is capable of receiving stimuli from the nervous system |
| Sarcoplasm | Intracellular material | Holds organelles and nutrients; provides the medium for glycolytic enzymatic reactions |
| Organelles | ||
| Myofibrils | Rodlike structures composed of smaller units called myofilaments: account for 80% of muscle volume | Contain contractile proteins (myofilaments), which are responsible for muscle contraction |
| T tubules | Series of tubules that run perpendicular (transverse) to the cell and are open to the external part of the cell | Spread polarization from the cell membrane into the interior of cell, which triggers the sarcoplasmic reticulum to release calcium |
| Sarcoplasmic reticulum | Interconnecting network of tubules running parallel with and wrapped around the myofibrils | Stores and releases calcium |
| Mitochondria | Sausage or spherical-shaped organelles; numerous in a muscle cell | Are the major site of energy production |
From Plowman SA, Smith DL: Exercise physiology for health, fitness, and performance, ed 2, San Francisco, 2003, Benjamin Cummings.
The muscle fiber contains the organelles found in other cells (including a large number of mitochondria) along with some specialized organelles. The organelles of specific interest are the transverse tubules, sarcoplasmic reticulum, and myofibrils. The myofibrils are composed of the protein myofilaments and are responsible for the contractile properties of muscles.
Sarcoplasmic Reticulum and Transverse Tubules
Figure 2-4 illustrates the relationship among the myofibrils, sarcoplasmic reticulum, and transverse tubules. The sarcoplasmic reticulum (SR), a specialized organelle that stores and releases calcium, is an interconnecting network of tubules running parallel with and wrapped around the myofibrils. (Note: in the figure the sarcolemma has been partially removed in order to illustrate the SR and myofibrils.) The major significance of the sarcoplasmic reticulum is its ability to store, release, and take up calcium and thereby control muscle contraction. Calcium is stored in the portion of the sarcoplasmic reticulum called the lateral sacs or cisterns.
Myofibrils and Myofilaments
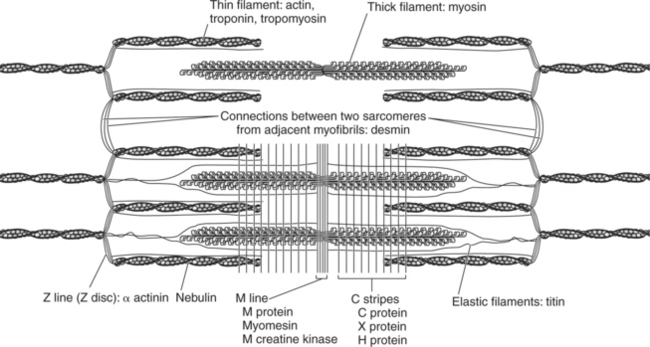
Each muscle fiber contains hundreds to thousands of smaller cylindrical units, or rodlike strands, called myofibrils (see Figure 2-4). Myofibrils are contractile structures composed of myofilaments. These myofibrils, or simply fibrils, typically lie parallel to the long axis of the muscle cell and extend the entire length of the cell. Myofibrils account for approximately 80% of the volume of a muscle fiber and are composed of two myofilaments (thick and thin myofilaments). Each repeating unit along the myofibril is referred to as a sarcomere.
Sarcomeres
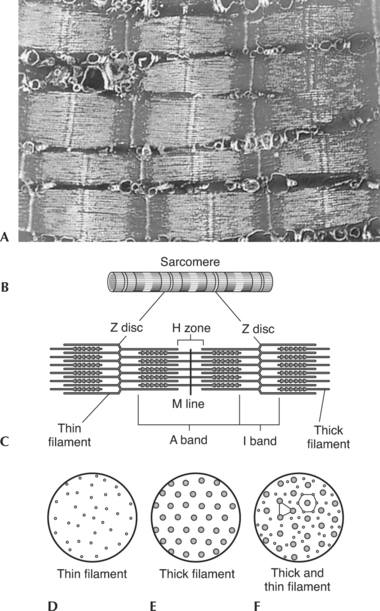
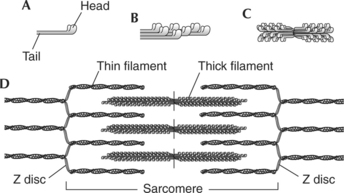
A sarcomere is the functional unit (contractile unit) of a muscle fiber. As illustrated in Figure 2-5, each sarcomere contains two types of myofilaments: thick filaments, composed primarily of the contractile protein myosin, and thin filaments, composed primarily of the contractile protein actin. Thin filaments also contain the regulatory proteins, troponin and tropomyosin. When myofilaments are viewed under an electron microscope, their arrangement gives the appearance of alternating bands of light and dark striations. The light bands are called I bands and contain only thin filaments. The dark bands are called A bands and contain thick and thin filaments, with the thick filaments running the entire length of the A band. Thus the length of the thick filament determines the length of the A band.
Myofilaments occupy three-dimensional space. The arrangement of the myofilaments at different points in the sarcomere is shown in Figure 2-5, D and F. Notice that in regions where the thick and thin filaments overlap, each thick filament is surrounded by six thin filaments and each thin filament is surrounded by three thick filaments.
A sarcomere consists of more than just contractile and regulatory proteins. Proteins of the cytoskeleton provide much of the internal structure of the muscle cell. Figure 2-6 diagrams the cytoskeleton of the sarcomere and its relationship to the contractile proteins.6 The M line and the Z disc hold the thick and the thin filaments in place, respectively. The elastic filament helps keep the thick filament in the middle between the two Z discs during contraction.
Molecular Structure of the Myofilaments
Thick Filaments
Thick filaments are composed primarily of myosin molecules (Figure 2-7). Each molecule of myosin has a rodlike tail and two globular heads (Figure 2-7, A). A typical thick filament contains approximately 200 myosin molecules.4 These molecules are oriented so that the tails form the central rodlike structure of the filament (Figure 2-7, B). The globular myosin heads extend outward and form cross-bridges when they interact with thin filaments. The myosin heads have two reactive sites: One allows it to bind with the actin filament, and one binds to ATP. Only when the myosin heads bind to the active sites on actin, forming a cross-bridge, does contraction occur.
The myosin subunits are oriented in opposite directions along the filament, forming a central section that lacks projecting heads (Figure 2-7, C). The result is a bare zone in the middle of the filament, which accounts for the H zone seen in the middle of the A band (Figure 2-7, D).
Thin Filaments
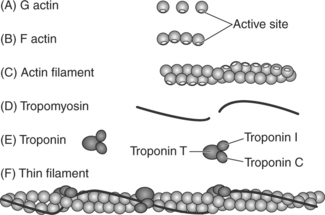
Thin filaments are composed primarily of the contractile protein actin. As illustrated in Figures 2-8, A and B, actin is composed of small globular subunits (G actin) that form long strands called fibrous actin (F actin). A filament of actin is formed by two strands of F actin coiled about each other to form a double helical structure; it resembles two strands of pearls wound around each other and may be referred to as a coiled coil (Figure 2-8, C). The actin molecules contain active sites to which myosin heads will bind during contraction.
The thin filaments also contain the regulatory proteins called tropomyosin and troponin, which regulate the interaction of actin and myosin. Tropomyosin is a long, double-stranded, helical protein that is wrapped about the long axis of the actin backbone (Figure 2-8, D). Tropomyosin serves to block the active site on actin, thereby inhibiting actin and myosin from binding under resting conditions.
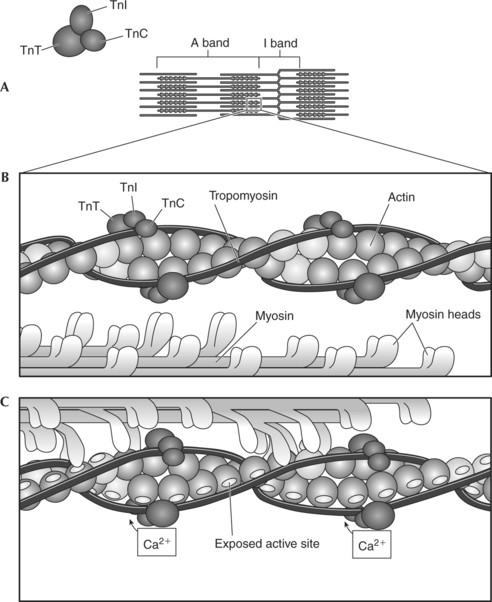
Troponin is a small, globular protein complex composed of three subunits that control the position of the tropomyosin (Figure 2-9). The three units of troponin are troponin C (Tn-C), troponin I (Tn-I), and troponin T (Tn-T). Tn-C contains the calcium-binding sites, Tn-T binds troponin to tropomyosin, and Tn-I inhibits the binding of actin and myosin in the resting state (Figure 2-9, B). When calcium binds to the Tn-C subunit, the troponin complex undergoes a configurational change. Because troponin is attached to tropomyosin, the change in the shape of troponin causes tropomyosin to be removed from its blocking position, thus exposing the active sites on actin.3,4 Once the active sites are exposed, the myosin heads can bind to the actin, forming the cross-bridges (Figure 2-9, C). Thus calcium is the key to controlling the interaction of the filaments and therefore muscle contraction.
Contraction of a Muscle Fiber
In order for a muscle to contract, an AP must be generated in the motor neuron that innervates the muscle fibers that will contract. The message from the motor neuron must then be passed to the muscle fiber through the neuromuscular junction. Finally, the AP must be conducted along the sarcolemma and into the interior of the muscle fiber to initiate movement of the myofilaments. The process whereby electrical events in the sarcolemma of the muscle fiber are linked to the movement of the myofilaments is called excitation-contraction coupling.
The Sliding-Filament Theory of Muscle Contraction
Excitation-Contraction Coupling
Figure 2-10 summarizes the events that occur during each phase of excitation-contraction coupling. Excitation-contraction coupling begins with depolarization and spread of an AP along the sarcolemma (point 2 in Figure 2-10) and continues with the propagation of the AP into the T tubules (point 2 in Figure 2-10). An AP in the T tubules causes the release of calcium from the lateral sacs of the sarcoplasmic reticulum (point 3 in Figure 2-10).
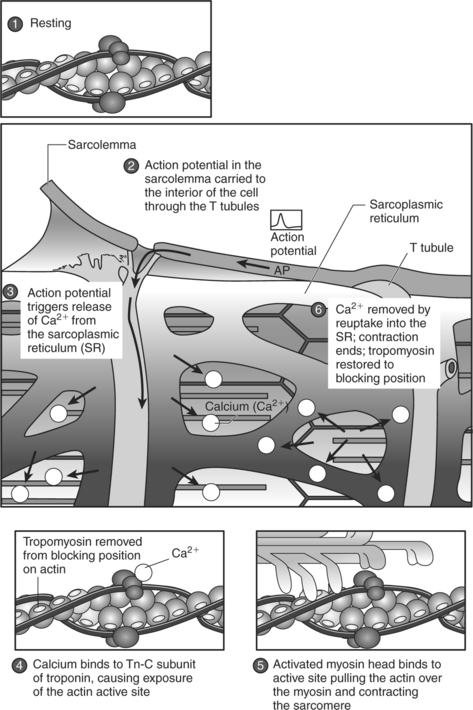
Figure 2-10 Phases of excitation-contraction coupling.
(From Plowman SA, Smith DL: Exercise physiology for health, fitness, and performance, ed 2, San Francisco, 2003, Benjamin Cummings.)
When calcium is released from the sarcoplasmic reticulum (the second phase), it binds to the troponin molecules on the thin filament. The binding of calcium to troponin causes troponin to undergo a configurational change, thereby removing tropomyosin from its blocking position on the actin filament (point 4 in Figure 2-10). The third phase of excitation-contraction coupling is the cross-bridging cycle (point 5 in Figure 2-10). The cross-bridging cycle describes the cyclic events that are necessary for the generation of force or tension within the myosin heads during muscle contraction. The generation of tension within the contractile elements results from the binding of the myosin heads to actin and the subsequent release of stored energy in the myosin heads. As shown in Figure 2-11, four individual steps are necessary for the cross-bridging cycle3,4,7:
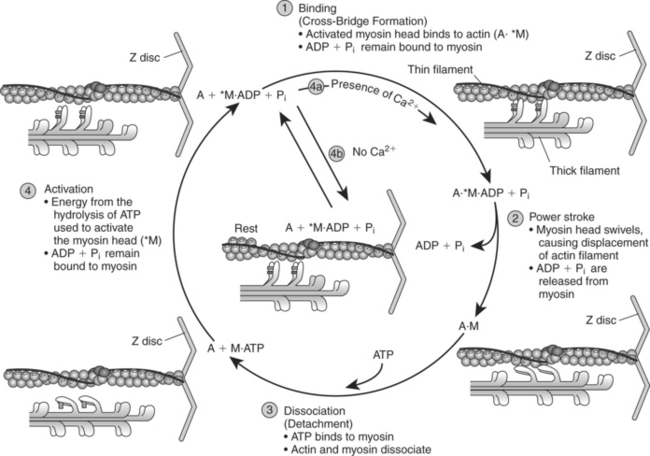
Figure 2-11 Force generation of the contractile elements: the cross-bridging cycle.
(From Plowman SA, Smith DL: Exercise physiology for health, fitness, and performance, ed 2, San Francisco, 2003, Benjamin Cummings.)
The first step in the cross-bridge cycle is the binding of activated myosin heads (*M) with the active sites on actin, forming cross-bridges. In Figure 2-11 a centered dot (·) is used to indicate binding, and an asterisk (*) is used to indicate activated myosin heads. Thus A·*M indicates that the activated myosin heads are bound to actin (A), whereas A + M indicates that actin and myosin are unbound.
The second step in the cross-bridging cycle is the power stroke. During this step, activated myosin heads swivel from their high-energy, activated position to a low-energy configuration (M with no *). This movement of the myosin cross-bridges results in a slight displacement (sliding) of the thin filament over the thick filament toward the center of the sarcomere. As shown in Figure 2-11, during the second step ADP and Pi are released from the myosin heads, resulting in myosin bound only to actin (A·M).
The cross-bridging cycle continues as long as ATP is available and calcium is bound to troponin (Tn-C), causing the active sites on actin to be exposed. On the other hand, activated myosin remains in the resting state awaiting the next stimulus if calcium is not available in sufficient concentration to remove tropomyosin from its blocking position on actin (see step 4b in Figure 2-11). Because each cycle of the myosin cross-bridges barely displaces the actin, the myosin heads must bind to the actin and be displaced many times for a single contraction to occur. Thus myosin makes and breaks its bond with actin hundreds or even thousands of times during a single muscle twitch. In order for this make-and-break cycle to occur, myosin heads must detach from actin and then be reactivated. This detaching and reactivating process requires the cycle to be repeated and requires the presence of ATP (see step 3).4 The analogy of a spring-loaded mousetrap may be helpful for understanding the role of ATP in providing energy to activate the myosin head. It takes energy to set the trap, just as it takes the splitting of ATP to set or activate the myosin head. Once set, however, the trap releases energy when it is sprung. In a similar manner, the myosin head possesses stored energy, which is released when the myosin heads bind to actin and swivel.
A review of Figure 2-11 reveals that ATP plays several important roles in muscle contraction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree