Arlen D. Hanssen
David G. Lewallen
Uncemented Hemispherical Cups in Extreme Bone Loss
INTRODUCTION
The failed acetabular component is frequently accompanied by severe osteolysis and large periacetabular bone loss. The challenge in reconstruction lies in clearly identifying the location and quality of remaining viable host bone, classifying the acetabular defect pattern, and choosing an appropriate reconstruction method and construct to gain an optimal outcome. Long-term success in acetabular reconstruction is obtained by maximizing the intimate contact of the implant with an adequate (typically >50%) amount of viable host bone and providing the necessary mechanical stability to allow successful osseointegration of the acetabular component. To date, this has been most reliably achieved with cementless, porous-coated implants,1–3 which have achieved 96% to 98% 10-year survivorship with aseptic loosening as the endpoint. However, a retrospective review of all uncemented acetabular components, including 2,443 revisions, performed at the Mayo Clinic over a 15-year period documented acetabular failure that increased at a steady and progressive rate after a decade of in vivo use (Fig. 14-1).4 In addition, the long-term success of the implant is dependent on the extent of bone loss and subsequent remaining host bone. The same institution reviewed 60 revision acetabular reconstructions with a first-generation cement-less hemispherical socket at a minimum of 5-year follow-up. A 12% failure rate was observed in those hips requiring <25% coverage from allograft and a disturbing 78% radiographic or clinical failure rate in 27 hips requiring >50% allograft coverage at the time of socket revision.4 Severe bone loss represents a challenging problem in the revision acetabular reconstruction. Bulk allograft and antiprotrusio cages have been the predominant reconstruction methods for severe acetabular bone loss; however, the results of these two reconstruction methods have been largely disappointing. The results of antiprotrusio cages in the literature document mechanical failure rates of up to 15% at midterm follow-up.5–9 Recent reports document success rates of only 64% to 76% with antiprotrusio cages used for large acetabular defects,8,10 with a high rate of complications, including sciatic nerve neurapraxias, loss of implant fixation, and failure of the cage by flange fracture.10 Acetabular reconstruction with structural allograft also is fraught with inconsistent and suboptimal results, with mechanical failure rates from 6% to 44%11–16 and up to 70% component loosening and migration in the most severe acetabular defects.14
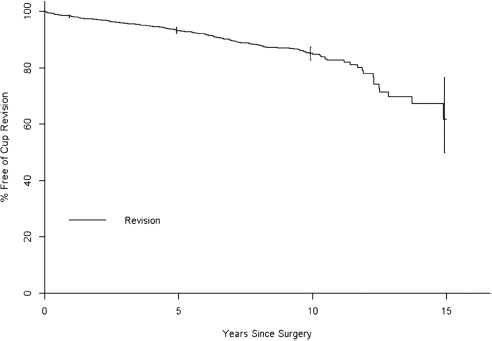
FIGURE 14-1. Survivorship curve of 2,443 revision uncemented acetabular components preformed at Mayo Clinic from 1984 through 1998. A progressive and nonlinear increase in component failure is seen, most pronounced after the first decade in vivo.
The decline in survivorship seen in cementless hemispherical components in revision acetabular reconstruction after the 10-year mark, and the inconsistent and suboptimal success of current techniques for reconstruction of large acetabular defects, serve as the impetus for the development of a new acetabular reconstruction method. Recently developed acetabular implants composed of a novel porous tantalum biomaterial have been used in conjunction with modular augments of identical material to create a single revision acetabular system with optimal biological and mechanical properties. The modularity of this system allows successful treatment of many severe bone defects encountered in revision acetabular surgery.
POROUS TANTALUM
A new highly porous tantalum biomaterial has recently been developed for application in hip and knee reconstruction surgery. Tantalum has been shown to be biocompatible via the response of osteoblasts in cell culture.17 The highly porous version of this material is produced by the deposition of commercially pure tantalum on a carbon skeleton of interconnecting pores (Fig. 14-2). This metal construct is extremely porous (75% to 80% porous by volume), compared to the 40% to 50% and 30% to 35% porosity of fiber-metal mesh and sintered-bead coatings, respectively.18,19 The higher fractional volume available for ingrowth with porous tantalum provides a more rapid development of interfacial shear strength. Histological analysis has demonstrated a 40% to 50% filling of pores with new bone at 4 weeks with a minimum interface fixation strength of 18.5 MPa, substantially higher than observed with other porous materials with less volumetric porosity.19
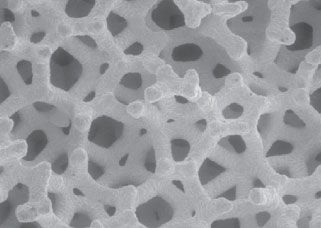
FIGURE 14-2. Scanning electron micrograph of porous tantalum biomaterial, illustrating the three-dimensional, open-pored structure.
In addition to rapid bone ingrowth and increased interface strength, porous tantalum provides the important bio-mechanical properties of increased material elasticity and a surface coefficient of friction greater than other implant surfaces. The coefficient of friction for porous tantalum on cancellous bone (0.88 to 0.98)20 is significantly greater than that previously reported for traditional porous-coated and sintered-bead materials (0.50 to 0.66)21. The increased coefficient of friction with porous tantalum likely provides greater initial implant stability, which is critical to obtaining long-term biological fixation. Furthermore, the modulus of elasticity of porous tantalum is in between cortical and cancellous bone, and is significantly less than titanium and chromium cobalt materials.22 This optimal elasticity of porous tantalum likely creates a more physiologic transfer of stresses to the periacetabular bone and theoretically decreases detrimental stress-shielding. Furthermore, the deformation of porous tantalum against hard cortical bone is attributable to its relative elasticity, a property that likely facilitates initial frictional stability and geometrical fit, while reducing the propensity for fracture upon complete implant seating.
In summary, the initial fixation provided by the porous tantalum coefficient-of-friction and decreased rigidity is supplemented by the rapid bone ingrowth and increased interface fixation strength to facilitate osseointegration. These material and biological properties of porous tantalum are likely beneficial in revision acetabular reconstruction, especially in the face of large acetabular defects or suboptimal bone quality.
REVISION ACETABULUM SYSTEM
The porous tantalum acetabular reconstruction system has evolved into a family of implants designed to address the vast majority of clinical situations and acetabular defects encountered in the revision setting. The revision porous tantalum acetabular shell is a hemispherical component that is composed almost entirely of porous tantalum (Fig. 14-3A,B). The shell is not uniformly hemispherical, however, and has a slightly elliptical shape with a larger diameter at the shell’s periphery to obtain adequate interference fit, which is further enhanced by the increased elasticity of the novel material as discussed previously. The revision shell has a multitude of screw holes from the manufacturer, and a polyethylene liner is cemented into the implant. In addition, a high-speed carbide burr can be used to easily bore through the porous tantalum material. This provides the opportunity to place acetabular screw holes in the revision shell in the direction of optimal bone stock, providing maximal initial implant stability and fixation. Cementation of a polyethylene liner into a metal shell has been shown to be equivalent to standard locking mechanisms in biomechanical testing,23 and when used with multiple appropriately placed acetabular bone screws, creates three-dimensional stability. This additional stability is obtained as the cement hardens around the acetabular screw heads and creates a fixed-angle or locking screw effect, analogous to the newly developed locking plates in orthopedic trauma surgery. Furthermore, early clinical follow-up of cemented liners into well-fixed acetabular components revealed no dissociation of the liner from the cement.24
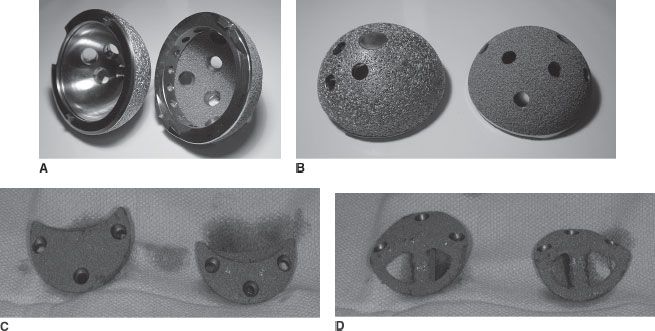
FIGURE 14-3. Porous-coated titanium (left) and porous tantalum (right) hemispherical acetabular components used in cementless revision acetabular reconstruction as viewed from the (A) concave and (B) outer convex surface. C,D: Modular porous tantalum augments of various sizes with fenestrations for supplemental bone graft.
In addition to the porous tantalum acetabular component designed for revisions, a modular trabecular metal shell has recently emerged with a locking mechanism for the polyethylene. This implant offers surgeons the advantages associated with modularity of the polyethylene liner; however, the inner titanium shell that lines the concave surface of the shell is rigid and imparts additional stiffness to the construct, rendering the porous metal material as a biologically friendly bone ingrowth surface without the benefits of elasticity for improved cup fixation. Furthermore, the surgeon is relegated to placing screws in the screw holes predetermined by the manufacturer, which may not be in the ideal position when attempting to gain fixation into deficient periacetabular bone.
A novel system of modular porous tantalum augments has been developed to address and manage the multitude of large osseous defects that are frequently encountered in revision acetabular reconstruction. Multiple shapes and sizes of these augments (Fig. 14-3C,D) accommodate the various acetabular bone defects encountered and compliment the various sizes of hemispherical acetabular components. The augments were developed and designed to obtain simultaneous biologic fixation and mechanical support to gain osseointegration of the uncemented hemispherical acetabular component to host bone. In addition, the augments are fenestrated to allow supplementation with morselized autograft or allograft. By obtaining mechanical and biological fixation, this porous tantalum augment system may allow the avoidance of structural allograft use, obviate the need for custom fabrication of implants, and provide a comprehensive system that facilitates the use of cementless hemispherical acetabular components by using the porous tantalum augments to address the wide variety of osseous defects encountered during these difficult reconstructions.
If available host bone is inadequate to obtain mechanical stability with the use of the porous tantalum augments and a hemispherical trabecular metal shell, an integrated porous tantalum cup-cage construct can be utilized. This construct employs the addition of an “antiprotrusio cage” inset and cemented to a porous tantalum revision shell that does not have sufficient mechanical stability by itself to obtain bone ingrowth. This construct allows the uncemented hemispherical cup to be placed in optimal orientation against the remaining, yet mechanically insufficient host bone. The goal is to obtain long-term bone ingrowth facilitated by the mechanical support imparted by the cage cemented into the trabecular metal revision shell. Furthermore, this cage-cup construct allows optimal socket positioning as the separate polyethylene liner is cemented into place once the hemispherical shell, morselized bone graft, and cage are secured with acetabular screws and cement. This cup-cage construct provides a treatment solution to the most severe and challenging acetabular defects that typically render a hemispherical acetabular component unstable. In addition, the cup-cage construct has application in the setting of impaired bone quality secondary to severe osteoporosis or prior irradiation.
INDICATIONS AND BENEFITS
The indications for acetabular revision include aseptic mechanical loosening, hip instability due to component malposition, and periprosthetic osteolysis associated with wear debris. An additional indication for revision total hip arthroplasty, albeit in a staged manner, is for periprosthetic infection necessitating removal and reimplantation of implants. Traditional porous-coated uncemented hemispherical titanium cups may be used for the vast majority of acetabular revisions with a high success rate, with 10-year survivorship rates of 96% to 98% with respect to aseptic loosening.1–3 However, as previously mentioned, disappointing results into the second decade of use in the revision setting with traditional porous-coated hemispherical implants4 (Fig. 14-1) has prompted the development of alternative implant materials, namely porous tantalum.
Relative contraindications to acetabular revision with a traditional porous-coated titanium hemispherical shell include acetabular osteonecrosis due to radiation exposure, pelvic discontinuity, and severe global acetabular bone loss precluding adequate apposition of the hemispherical implant to viable host bone. However, it should be emphasized that these contraindications are relative, and the biological and mechanical characteristics of porous tantalum demonstrate some theoretical advantages in these challenging clinical settings and have been used with initial short-term success.
Inherent in these indications and contraindications are the requisites for success with a cementless hemispherical acetabular implant in the revision setting. These requirements include an adequate amount of viable host bone contact to provide sufficient mechanical stability and the appropriate biological conditions to allow bone ingrowth and long-term clinical success. Although currently unsubstantiated by clinical or biomechanical studies, it is commonly accepted that at least 50% implant contact with host bone improved the likelihood of subsequent implant osseointegration. It is probable that the ability to achieve adequate mechanical fixation is additionally related to the specific location of viable host bone, the quality of host bone, and the biologic activity of the remaining bone in contact with the prosthesis. Early results suggest that the properties of porous tantalum may provide sufficient mechanical stability of the hemispherical implant in the setting of more severe bone deficiencies. For example, the increased coefficient of friction and increased elasticity of porous tantalum material may allow achievement of mechanical stability with a hemispherical implant in the setting of <50% host bone contact. Furthermore, the biologically friendly porous macrostructure may allow osseointegration for long-term stability in the setting of limited host bone contact, poor bone quality, or previously irradiated bone. Biomechanical and long-term clinical studies are required to confirm these potential benefits.
The indications for a porous tantalum cup-cage construct are similar to those for standard antiprotrusio cages. The typical indications include large irregular acetabular defects and/or a small acetabular AP dimension that renders a hemispherical cup unstable, major segmental defects in the posterior column or dome, pelvic discontinuity, or pathologic bone such as seen with prior radiation exposure. Unlike standard cages, which provide purely mechanical fixation and may be subjected to increased loads over time, a porous tantalum cup-cage construct allows for bone ingrowth to the porous metal that subsequently provides a gradual decrease in stresses applied to the iliac and ischial flanges and screws. This should decrease the potential for late fatigue fracture, loosening, and failure.
POROUS TANTALUM ACETABULAR CONSTRUCT CLASSIFICATION
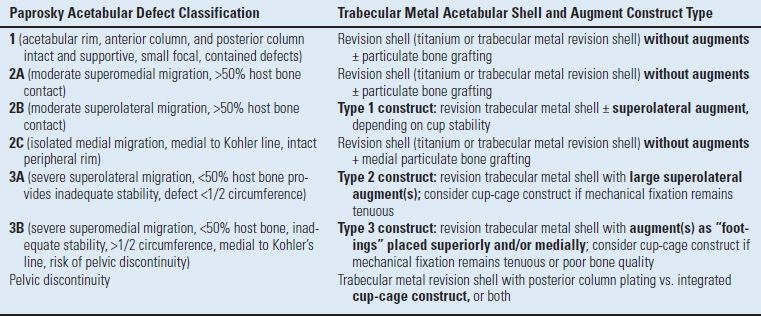
Accurate radiographic and intraoperative classification of acetabular defects and identification of viable host bone is essential to enacting the appropriate treatment algorithm using the modular porous tantalum acetabular augment system. The authors favor the Paprosky classification25 of acetabular defects, as it has proven beneficial in ascertaining and enacting appropriate treatment plans in acetabular revision surgery. In addition, the Paprosky classification system correlates well with the porous tantalum acetabular construct classification system developed by the authors to describe and facilitate the appropriate porous tantalum construct. The modular augment construct classification system (Table 14.1) provides a perioperative decision-making algorithm and guide that facilitates application of the various augment sizes and positions to maximize hemispherical implant contact to host bone and provide the required mechanical stability.
TABLE 14.1 Modular Trabecular Metal Acetabular Augment Construct System
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








