Chapter 12 Typical Atrial Flutter
Pathophysiology
Right Atrial Anatomy
The posterior smooth-walled RA and the anterolateral trabeculated RA are separated by the crista terminalis on the lateral wall and the eustachian ridge in the inferior aspect. The sulcus terminalis, where the sinus node is located, is a subtle groove on the epicardial surface of the heart corresponding to the crista terminalis. The crista terminalis is a C-shaped, convex, thick muscular ridge that runs from the high septum, anterior to the orifice of the SVC superiorly, and courses caudally along the posterolateral aspect of the RA. In its inferior extent, it courses anteriorly to the orifice of the IVC. As the crista reaches the region of the IVC, it is extended by the eustachian valve ridge. The eustachian valve is the remnant of the embryonic sinus venosus valve, which manifests as a flap of variable thickness and mobility along the orifice of the IVC; this valve can continue as a ridge superiorly along the floor of the RA to the ostium of the CS (CS os), to join the valve of the CS, form the tendon of Todaro, and then continue onto the interatrial septum as the inferior limbus of the fossa ovalis.1
The tricuspid annulus lies anterior to the body of the RA, and its inferior portion lies a short distance (approximately 1 to 4 cm) anterior to the eustachian ridge, although its course varies among individuals.2 The cavotricuspid isthmus (CTI) is the part of the RA between the ostium of the IVC and the eustachian ridge (posteriorly) and the tricuspid annulus (anteriorly). The CTI runs in an anterolateral-to-posteromedial direction, from the low anterior RA to the low septal RA (see Fig. 17-1). Its width and muscle thickness are variable, from a few millimeters to more than 3 cm in width and more than 1 cm in depth. The CTI becomes wider in a medial-to-lateral direction, and it is thinnest in its central portion. A thick eustachian ridge (greater than 4 mm) is seen in 24% of patients. At mid-diastole, the central isthmus is straight in 8% of patients, concave in 47% of patients, and pouch-like (more than 5 mm) in 45% of patients.3,4 The eustachian ridge (often composed of partly or largely fibrous tissue) occurs as an elevation on the CTI. The area between the tricuspid annulus and the eustachian ridge is referred to as the subeustachian isthmus, whereas the downslope of the eustachian ridge leads to the junction of the RA and IVC. The pectinates, as they fan out from the crista terminalis or other muscle bundles on the CTI, typically spare the myocardium just atrial to the tricuspid valve. This smooth portion of the cavotricuspid annulus is referred to as the vestibular portion.1 In the normal heart, CTI anatomy can be flat, “hilly” (from prominent eustachian ridge or pectinate muscles, or both), concave, or have a pouch-like recess.
Typical Atrial Flutter Circuit
Typical atrial flutter (AFL) is a type of macroreentrant atrial tachycardia (AT) that uses the CTI as an essential part of its circuit. The circuit boundaries are the tricuspid annulus, crista terminalis, IVC orifice, eustachian ridge, CS os, and probably fossa ovalis. These barriers (lines of conduction block) can be functional or anatomical and are necessary to provide adequate path length for the flutter reentry circuit. Whereas the anterior boundary of the tachycardia circuit has been well established as being the tricuspid ring, the posterior boundaries are more complex and not as well defined, and they occur at a variable distance from the anterior border; the posterior borders are narrowest in the region of the eustachian ridge and widest in the anterior part of the RA.2,5–8
The CTI provides the protected zone of slow conduction necessary for the flutter reentry circuit. The area of slowest conduction is probably localized in the lateral aspect of the CTI in younger patients and in the medial aspect in older patients.9 Conduction velocity in the CTI during pacing in sinus rhythm is slower in patients with typical AFL compared with those without any history of AFL.3,10 The mechanism of the slower conduction velocity in the CTI, relative to the interatrial septum and RA free wall, is uncertain but can be related to the anisotropic fiber orientation. With aging or atrial dilation, intercellular fibrosis can change the density of gap junctions and produce nonuniform anisotropic conduction through the trabeculations of the CTI. Additionally, the CTI and RA in patients with typical AFL are significantly larger than those in a control population.6
The crista terminalis plays an important role as a functional barrier during typical AFL. Conduction delay and rate-related transverse block across the crista terminalis has been consistently observed in sinus rhythm and during pacing in humans. A line of transverse conduction block along the crista terminalis serving as a lateral boundary can be determined by the presence of double and split potentials recorded during AFL or rapid pacing from either side of the crista terminalis during electrophysiological (EP) testing. Structural characteristics of the crista terminalis influence transverse conduction; steep slope and arborization of the crista terminalis have been implicated as geometric factors in its transverse conduction block. Typical AFL is more likely to occur in the setting of a thicker and continuous crista terminalis, and these patients are more likely to exhibit transverse crista terminalis conduction block at longer cycle lengths (CLs) as opposed to controls. Similarly, the region posterior to the crista terminalis (the posterior smooth-walled RA) also has been shown to demonstrate functional transverse conduction block during AFL or rapid pacing.11,12
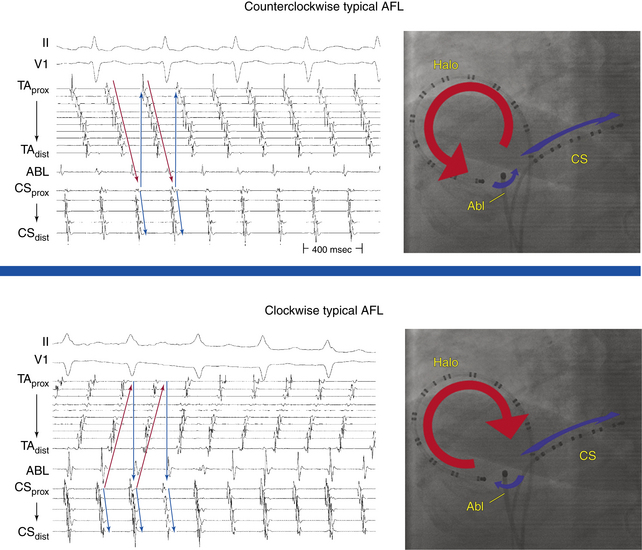
Typical AFL is of two types: counterclockwise and clockwise.5 In counterclockwise AFL, the activation wavefront propagates caudocephalically up the septal side of the tricuspid annulus toward the crista terminalis and advances cephalocaudally along the lateral wall of the RA to reach the lateral tricuspid annulus, after which it propagates through the CTI (“counterclockwise” as viewed in the left anterior oblique [LAO] view from the ventricular side of the tricuspid annulus). The width of the activation wavefront in typical AFL varies considerably, determined by the distance between the anterior and posterior boundaries at any given part of the circuit. It is very narrow inferiorly at the CTI and substantially wider moving upward. The substantial distance between anterior and posterior borders as well as the anatomical barriers superiorly, combined with variability in the completeness of the posterior border, creates conditions for substantial variability in the upper part of the circuit. Despite a relatively similar activation sequence, the active circuit (as determined by entrainment mapping) is variable. Most commonly, the reentrant wavefront courses not around the tricuspid annulus but obliquely between anterior and posterior borders away from the tricuspid annulus along any available, more rapidly conducting segments. Consequently, significant portions of the RA, including areas around the tricuspid annulus, can often be passively activated. In many subjects, the upper portions of the circuit pass behind the RA appendage and lie near or at the posterior circuit border, or they bifurcate around the SVC or RA appendage, or both. The posterior border can extend completely or partially between the IVC and the SVC.7,8
The flutter circuit is entirely confined within the RA. Left atrial (LA) activation occurs as a bystander and follows transseptal conduction across the inferior CS-LA connection, Bachmann bundle, or fossa ovalis.2,6
In clockwise (reverse typical) AFL, activation propagates in the direction opposite to that in counterclockwise typical AFL (Fig. 12-1). Clockwise AFL is observed in only 10% of clinical cases, despite the fact that it is easily inducible in the EP laboratory with programmed electrical stimulation. Clockwise AFL can be induced in the EP laboratory in approximately 50% of patients who clinically present with only counterclockwise AFL. The 9:1 clinical predominance of counterclockwise AFL can be related to the localization of an area with a low safety factor for conduction in the CTI, close to the atrial septum. Additionally, counterclockwise AFL is more likely to be induced with rapid atrial pacing from the CS os. Conversely, clockwise AFL is more likely to be induced with pacing from the low lateral RA pacing. These observations may be related to the anisotropic properties of the CTI and the development of rate-dependent conduction delays and unidirectional block necessary for tachycardia induction, which may be affected by the site of stimulation.2,6 Spontaneous AFL initiation may be related to rapid bursts of pulmonary vein discharges (atrial fibrillation [AF]).
Double-Wave Reentry
Double-wave reentry is manifest by acceleration of the tachycardia rate but with identical surface and intracardiac electrogram morphology. It can be recognized by the simultaneous activation of the superior and inferior regions of the tricuspid annulus, with all activation being sequential. This rhythm rarely lasts for more than a few beats and can serve as a trigger for AF. Because the CTI is still a necessary part of the circuit, double-wave reentry is amenable to CTI ablation.2,6
Clinical Considerations
Epidemiology
It is estimated that the overall incidence of AFL in the United States is 88 per 100,000 person-years. AFL accounts for approximately 15% of supraventricular arrhythmias and frequently coexists with or precedes AF. Although in clinical practice AFL appears to be less common than paroxysmal supraventricular tachycardia, population-based data show that in the general population, AFL is diagnosed for the first time more than twice as often. Adjusted for age, the incidence of AFL in men is more than 2.5 times that of women. Paroxysmal AFL can occur in patients with no apparent structural heart disease, whereas chronic AFL is usually associated with underlying heart disease, such as valvular or ischemic heart disease or cardiomyopathy. At highest risk of developing AFL are men, older adults, and individuals with preexisting heart failure or chronic obstructive lung disease. In approximately 60% of patients, AFL occurs as part of an acute disease process, such as exacerbation of pulmonary disease, following cardiac or pulmonary surgery, or during acute myocardial infarction.13
Principles of Management
Acute Management
Acute therapy for patients with AFL depends on the clinical presentation and may include cardioversion and the use of AVN blockers to slow the ventricular rate during the AFL. Cardioversion (electrical or chemical) is commonly the initial treatment of choice. Electrical cardioversion is almost always successful in terminating AFL, and it often requires relatively low energies (less than 50 J). Chemical cardioversion can be achieved with intravenous ibutilide in 38% to 76% of cases, and this agent is more effective than intravenous amiodarone, sotalol, and class IC agents. Overdrive atrial pacing (via a catheter in the esophagus or the RA) can effectively terminate typical AFL, but it can also induce conversion of AFL into AF. Anticoagulation in the pericardioversion period should be considered and is guided by the duration of the AFL and the patient’s stroke risk factors, by using the same criteria as for AF (see Chap. 15).14
Chronic Management
When AFL occurs as part of an acute disease process, long-term therapy of the arrhythmia is usually not required after sinus rhythm is restored and the underlying disease process is treated. The long-term success rate of antiarrhythmic drugs to prevent AFL recurrence appears to be limited, and complete suppression of AFL can be difficult to achieve. On the other hand, ablation is highly successful at the conclusion of a relatively short and low-risk procedure. Therefore, catheter ablation of the CTI is the treatment of choice for typical AFL, whether paroxysmal or persistent, and long-term drug therapy is rarely indicated and should be reserved for unusual circumstances.2
Several antiarrhythmic drugs have demonstrated efficacy in suppression of AFL, including class IA (quinidine, procainamide, and disopyramide), class IC (flecainide and propafenone), and class III (sotalol, amiodarone, dofetilide, and dronedarone) agents. In the absence of structural heart disease, class IC agents are the drugs of choice. The use of antiarrhythmic agents should be instituted in conjunction with AVN blockers to avoid the risk of rapid ventricular rates secondary to the vagolytic effects of class I drugs and slowing of the flutter rate.14
Electrocardiographic Features
P Waves
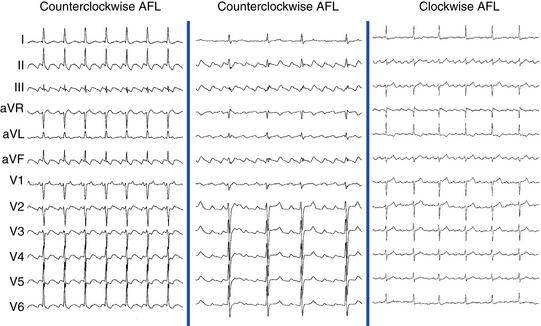
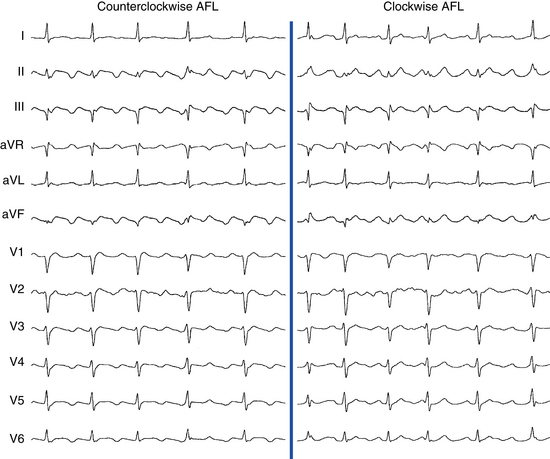
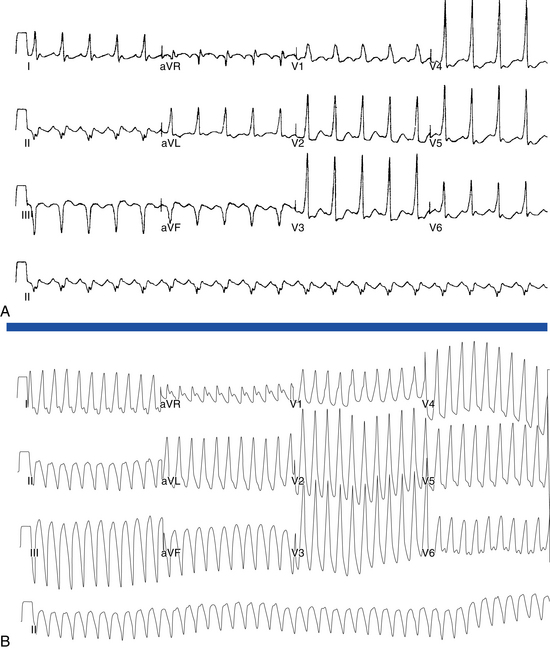
Flutter waves appear as atrial complexes of constant morphology, polarity, and CL. Typically, flutter waves are most prominent in the inferior leads (II, III, aVF) and lead V1. In the inferior leads, they resemble a picket fence (sawtooth) because the leads are primarily negative. This consists of a downsloping segment, followed by a sharper negative deflection, and then a sharp positive deflection, with a positive overshoot leading to the next downsloping plateau. The relative size of each component can vary markedly.2
Counterclockwise AFL (Fig. 12-2) can be characterized by pure negative deflections in the inferior leads, negative and then positive deflections that are equal in size, or a small negative and then a larger positive deflection. Those three varieties coexist with tall positive, small positive, or biphasic P waves in lead V1, respectively. With progression across the precordium, the initial component rapidly becomes inverted and the second component isoelectric usually by lead V2 to V3. This produces the overall impression of an upright flutter wave in lead V1, which becomes inverted by lead V6. A negative deflection always precedes the positive deflection in the inferior leads in counterclockwise AFL, and the degree of positivity in the inferior leads appears to be related to the coexistence of heart disease and LA enlargement. Lead I is low-amplitude isoelectric, and lead aVL is usually upright.15
The surface ECG appearance of clockwise typical AFL is more variable than that of counterclockwise typical AFL, but in many respects, clockwise AFL presents an inversion of the appearance in counterclockwise AFL. Clockwise AFL generally has broad positive deflections in the inferior leads, with characteristic notching (see Fig. 12-2).2 However, there is an inverted component preceding the upright notched component. Depending on the amplitude of this component, the appearance can be of continuous undulation without an obviously predominant upright or inverted component. On other occasions, it may appear that the inverted component is dominant, thus superficially mimicking counterclockwise AFL. Lead V1 is characterized by a wide negative and usually notched deflection. There is transition across the precordium to an upright deflection in lead V6. Lead I is usually upright, and lead aVL is low-amplitude negative and notched.15
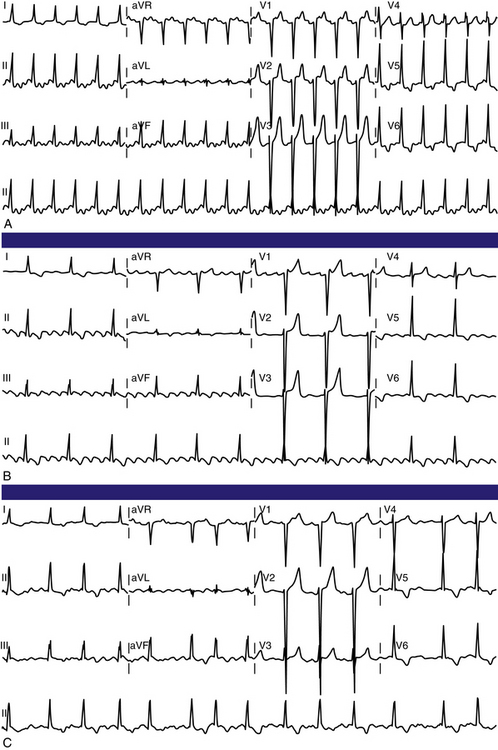
Typical AFL usually has an atrial rate of 240 to 340 beats/min. However, AFL can be slower in patients receiving antiarrhythmic agents or after incomplete CTI ablation (Fig. 12-3), whereby flutter CLs as long as 450 milliseconds have been observed. If the ventricular response is half the atrial rate, it can be difficult to identify flutter waves “buried” within the QRS or T waves (Fig. 12-4). Close inspection of the QRS and T waves, and comparisons with ECGs obtained in normal sinus rhythm, can help identify buried flutter waves. Furthermore, vagal maneuvers and AVN blockers can slow AV conduction and unmask the flutter waves.
Atrioventricular Conduction
Most commonly, 2:1 AV conduction is present during AFL. Variable AV conduction and larger multiples (e.g., 4:1 or 6:1) are not uncommon. Slowing the atrial rate during AFL caused by antiarrhythmic drugs or following a prior incomplete CTI ablation can result in a paradoxical increase in the ventricular rate caused by better AVN conduction of the slower flutter beats (Fig. 12-5). Rapid 1:1 AV conduction is most commonly seen in patients with anterogradely conducting bypass tracts (Fig. 12-6), but it may also be present in cases of enhanced AVN conduction secondary to high sympathetic tone (e.g., exercise, sympathomimetic drugs).2
QRS Morphology
The QRS complex during AFL is often identical to that during sinus rhythm. However, flutter beats can be aberrantly conducted because of functional bundle branch block, most frequently right bundle branch block (see Fig. 12-5). Even with normal ventricular conduction, the QRS complex may be slightly distorted by temporal superimposition of flutter waves on the QRS complex. Thus, the QRS complex can appear to acquire a new or larger R, S, or Q wave.2
Electrophysiological Testing
Typically, a decapolar catheter (positioned into the CS with the proximal electrodes bracketing the CS os) and a multipolar (20 or 24 pole) Halo catheter (positioned at the tricuspid annulus) are used to map typical AFL. The distal tip of the Halo catheter is positioned at 6 to 7 o’clock in the LAO view, so that the distal electrodes will record the middle and lateral aspects of the CTI, the middle electrodes will record the anterolateral RA, and the proximal electrodes may record the RA septum (depending on the catheter used and RA size). Instead of the Halo and CS catheters, some laboratories use a single duodecapolar catheter around the tricuspid annulus, thus extending the catheter tip inside the CS. Such a catheter can straddle the CTI and provide recording and pacing from the medial and lateral aspects of the isthmus, assuming good catheter-tissue contact at these locations. In the latter arrangement, however, the body of the duodecapolar catheter crossing over the CTI can potentially hinder manipulation and positioning of the ablation catheter tip to achieve adequate tissue contact for effective ablation.
Induction of Tachycardia
AFL can be induced readily with programmed electrical stimulation in most patients with a clinical history of AFL. Reproducible initiation of counterclockwise AFL is possible in more than 95% of patients.16 Rapid atrial pacing is more likely to induce AFL than a single AES, but as likely as introducing two AESs. On the other hand, the frequency of single or double AESs initiating AFL is low in patients without a history of AFL (less than 10%). Counterclockwise AFL is more likely to be induced by stimulation from the CS os; conversely, clockwise AFL is more likely to be induced with low lateral RA pacing. Induction of AFL usually occurs once unidirectional CTI block develops during pacing (Fig. 12-7). The faster the pacing rate and the shorter the AES coupling intervals, the more likely it will be that AF is induced, which is usually self-terminating but can be sustained in less than 10% of patients with no clinical history of AF. The significance of induction of AF in these patients is uncertain.
Tachycardia Features
The presence of anterogradely conducting bypass tracts with a short refractory period can result in preexcited AFL with rapid 1:1 AV conduction. Infusion of isoproterenol can enhance AVN function and occasionally facilitate 1:1 AV conduction, especially when the atrial rate is relatively slow.2 Adenosine increases the degree of AV block, but it also shortens atrial refractoriness and can result in the degeneration of AFL into AF.
Diagnostic Maneuvers during Tachycardia
Atrial Extrastimulation During Atrial Flutter
An AES commonly results in resetting of the AFL circuit.16 The closer the site of atrial stimulation is, the easier the resetting of the AFL circuit at longer coupling intervals will be. AFL has a resetting response pattern typical of reentrant circuits with fully excitable gaps: flat (for approximately 15% to 30% of the tachycardia CL, equal to approximately 30 to 63 milliseconds in the absence of drugs, and up to 100 milliseconds with class I antiarrhythmic agents) and then an increasing return CL with progressively shorter coupling intervals. The ability to capture the atrium without affecting (resetting) the AFL circuit timing indicates that the pacing site is outside the AFL circuit (e.g., RA appendage or distal CS).
It is usually difficult for a single AES to terminate AFL because AFL has a sizable fully excitable gap (15% to 30% of the tachycardia CL) that makes it difficult for a single AES to penetrate the AFL circuit with adequate prematurity to terminate the AFL without intervening atrial refractoriness and intraatrial conduction delays. An AES delivered in the region of the CTI has the greatest chance of terminating AFL because it can capture the isthmus tissue with a very short coupling interval (close to the ERP of this critical site), given the lack of intervening tissue between the stimulation site and isthmus. Termination of AFL always occurs because of conduction block in the CTI.
Atrial Pacing During Atrial Flutter
Entrainment
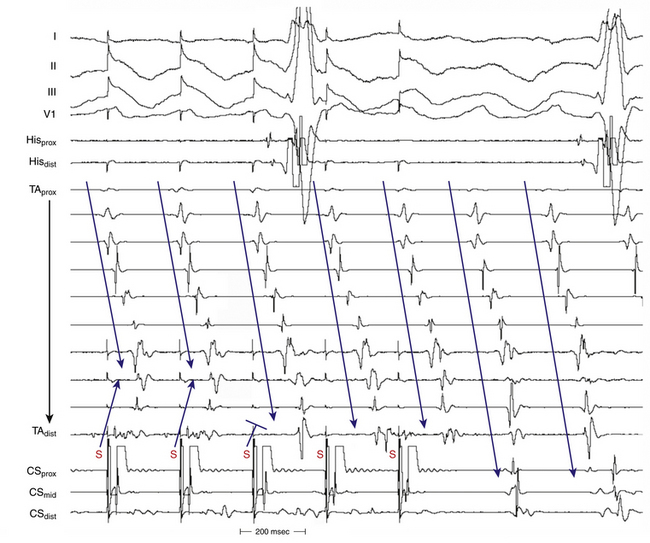
Overdrive atrial pacing at long CLs (i.e., 10 to 30 milliseconds shorter than the tachycardia CL) can almost always entrain typical AFL. The slower the pacing rate and the farther the pacing site from the reentrant circuit, the longer the pacing drive will need to be to penetrate and entrain the tachycardia. As discussed in detail in Chapter 13, achievement of entrainment of the AT establishes a reentrant mechanism of the tachycardia and excludes triggered activity and abnormal automaticity as potential mechanisms. However, it is important to understand that the mere acceleration of the tachycardia to the pacing rate and then resumption of the original tachycardia after cessation of pacing do not establish the presence of entrainment. After cessation of each pacing drive, the presence of entrainment should be verified by demonstrating the presence of fixed fusion of the paced complexes at a given pacing CL, progressive fusion at faster pacing CLs, and resumption of the same tachycardia morphology following cessation of pacing with a nonfused complex at a return cycle equal to the pacing CL. During entrainment of AFL, fusion of the stimulated impulse can be observed on the surface ECG, but it is easier to recognize on intracardiac recordings from the Halo and CS catheters. Entrainment with manifest fusion can be demonstrated with pacing from sites outside the CTI, such as lateral RA and CS. Conversely, pacing at the CTI results in entrainment with concealed fusion, whereby P waves (on the surface ECG and intracardiac recordings) during pacing are identical to those during the tachycardia.17,18
Termination
More rapid atrial burst pacing (pacing CL 20 to 50 milliseconds shorter than the AFL CL) results in termination of AFL in most cases.16 Termination of AFL during rapid pacing can be indicated by a sudden change of P wave morphology on the surface ECG and by a change of atrial activation sequence in the HB and CS os recordings. This is seen particularly with high RA pacing during counterclockwise AFL, whereby on termination of AFL, the negative flutter waves in the inferior leads change suddenly into upright P waves, thus reflecting a change in the atrial activation sequence to one of high RA pacing (i.e., simultaneous RA lateral and septal activation in a craniocaudal direction).5 However, if the pacing site is distant from the AFL circuit (e.g., distal CS), a large mass of the atrial tissue can be captured by the pacing stimulus, to produce a marked change in P wave morphology (i.e., manifest fusion) without terminating the AFL. Failure to terminate AFL with rapid pacing can be caused by any of the following: (1) a short period of pacing or pacing at a relatively long CL—the closer the pacing CL is to the tachycardia CL, the longer the pacing duration will need to be to terminate the tachycardia; (2) a pacing site distant from the AFL circuit, with the intervening atrial tissue preventing penetration of the AFL circuit; or (3) the possibility that an apparent AFL on the ECG may actually be AF with streaming of the RA activation wavefront or may be a focal nonreentrant AT.5,16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree