Chapter 11 Tumor and Tumor-like Conditions
Radiographs
Even with the availability of advanced imaging techniques, radiographs are extremely important and serve as the appropriate starting point for imaging of musculoskeletal tumors, particularly osseous tumors. As such, it is critical for the physician interpreting radiographs to have an understanding of radiographic appearances. This knowledge will aid in the decision-making process to determine when further workup is needed and when lesions can be left alone. Location and patient age are the two most important features when a differential diagnosis is created. Bone tumor location is described by both longitudinal location (i.e., diaphyseal, metaphyseal, or epiphyseal) and transverse location (i.e., medullary, cortical, or juxtacortical), as well as by the nature of cortical or surface involvement.56
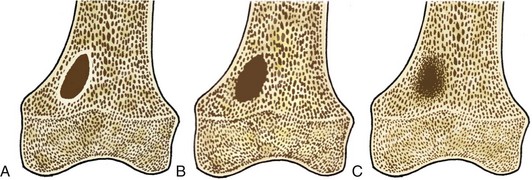
Tumors are described radiographically as having a narrow or a wide transition zone.50 A narrow transition zone is defined as one that can be traced with a pencil, with all the borders well visualized. Tumors with narrow transition zones are slow-growing, although not always benign. A wide transition zone on the contrary is ill-defined, with a border that is indistinct. Tumors with wide transition zones are aggressive and are more commonly malignant. Because a wide transition zone encompasses both larger, discrete lesions and lesions with very ill-defined borders, tumors may be further subclassified into three categories: (1) geographic, which is further divided into subtypes 1A, 1B, and 1C; (2) moth-eaten; and (3) permeative. Although there is some overlap, the likelihood of a lesion being malignant increases as one moves along the spectrum from geographic to permeative.48
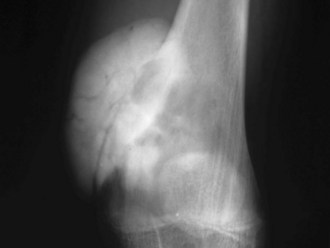
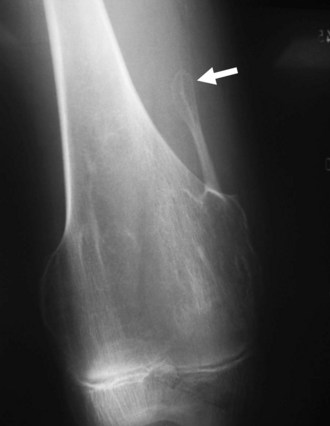
Geographic 1A lesions are those with a thin, well-defined sclerotic border. Examples of geographic 1A lesions are bone cysts, nonossifying fibromas, and benign cartilaginous lesions such as chondroblastomas and enchondromas. Geographic 1B lesions have thin, well-defined, and nonsclerotic margins. The aforementioned lesions in category 1A fit here as well; however, lesions such as giant cell tumors and metastases are also included. Geographic 1C lesions have ill-defined margins (wide transition zones) and typically, although not always, are more aggressive. Metastases, osteosarcomas, and chondrosarcomas are examples of type 1C lesions. The terms “moth-eaten” and “permeative” are often used interchangeably, and although they both connote aggressive processes, their appearances differ slightly (Fig. 11-1). Type 2, or moth-eaten, lesions have multiple, tiny, ill-defined lucencies that are discernible from one another. Type 3, or permeative, lesions have multiple, tiny, ill-defined lucencies that are indiscernible from one another and instead give the appearance of vague diffuse lucency throughout the bone. The types of lesions in each category are essentially the same and include mainly malignant processes such as Ewing’s sarcoma, osteosarcoma, osteomyelitis, and some metastases. Metabolic conditions such as hyperparathyroidism cause a permeative rather than moth-eaten appearance48 (Fig. 11-2).
It is also important to understand the different internal matrices that bone tumors produce, which are best divided into mineralized and nonmineralized. The two types of mineralized bone matrix are osteoid, which is cloudy and dense, and chondroid, which is typically described as flocculent or “ring and arc” (Fig. 11-3). Nonmineralized matrix may have a lucent or ground glass appearance.83
Periosteal reaction often accompanies bone tumors and is an important imaging feature to help differentiate between aggressive and nonaggressive tumors. Typically, benign tumors stimulate slow periosteal growth with periosteal reaction types such as solid or buttressing. Aggressive tumors cause aggressive periosteal reactions, with subtypes such as lamellated, “hair-on-end,” and sunburst. It should be noted that benign tumors such as Langerhans’ cell histiocytosis and chondroblastoma can cause aggressive periosteal reaction.42
CT/MRI
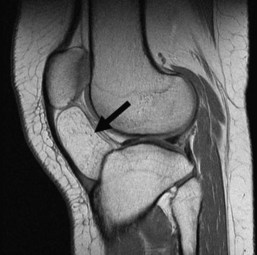
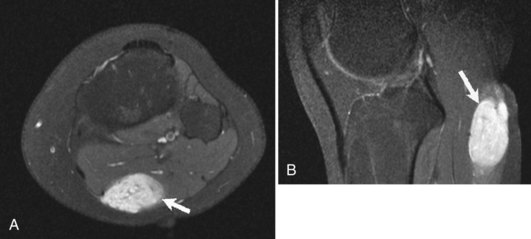
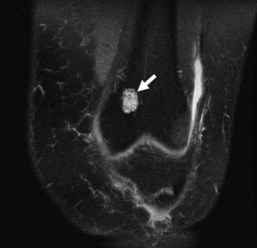
Although radiographs play a major role in the workup of musculoskeletal tumors, CT and MRI are useful for diagnosing some osseous and soft tissue lesions. Both CT and MRI can be used to categorize the internal matrix of bony lesions; CT is preferable for evaluating types of internal calcification (chondroid vs. osteoid), whereas the better contrast resolution of MRI affords it an advantage in determining the overall tissue type (i.e., fibrous vs. cartilaginous). CT and MRI are useful for determining cortical involvement of bony tumors, although CT offers better spatial resolution and better ability to detect cortical abnormalities. On MRI, the cortex demonstrates signal void on all sequences, which affords excellent negative contrast when there is tumor involvement of the cortex. CT and MRI are also useful for narrowing the differential diagnosis of primary soft tissue lesions and malignant osseous lesions with soft tissue extension. Both modalities can be diagnostic for soft tissue lesions with specific imaging characteristics such as lipomas, certain vascular tumors, and pigmented villonodular synovitis (PVNS); biopsy can often be avoided as a result58 (Fig. 11-4). Overall, CT and MRI are equally accurate for assessment of local staging of soft tissue and bone tumors, although MRI is preferable because of its soft tissue contrast and lack of radiation (Fig. 11-5).69 Either modality can be used to assess response to therapy, with imaging usually performed 3 to 4 months after treatment to allow for resolution of reactive changes.55 Rare superficial sarcomas such as dermatofibrosarcoma protuberans can be evaluated with ultrasound by the experienced musculoskeletal sonographer.
MRI and CT are useful for preoperative assessment and staging of bone and soft tissue tumors. MRI is superior to CT for assessing the intramedullary extent of osseous lesions by demonstrating the extent of reactive marrow edema. MRI is also superior to CT for evaluation of joint involvement, which is a crucial factor in determining whether extra-articular limb salvage is necessary. Both CT and MRI are excellent for evaluation of cortical breakthrough and soft tissue extension, which are important factors in the staging of osseous tumors. For bone tumors with soft tissue extension and primary soft tissue tumors, MRI is the best modality for evaluation of potential neurovascular involvement. Tumor surrounding the neurovascular bundle is diagnostic for neurovascular bundle tumor involvement, whereas the presence of a fat plane is diagnostic for absence of neurovascular bundle involvement. Absence of a fat plane between the neurovascular bundle and the lesion means that neurovascular involvement cannot be excluded.58 Finally, MRI is preferred over CT in evaluating for local tumor extension and spread of disease to regional lymph nodes.
CT is preferable to MRI when the matrix is not well evaluated on radiography, when the tumor is in an anatomic location not suited for MRI (i.e., fibula, ribs, sternum), or when an MRI cannot be performed. Whether CT or MRI is used, contrast is recommended primarily for two reasons when working up a musculoskeletal tumor: (1) to determine whether a lesion is cystic or solid, and (2) to determine nodularity or recurrent tumor in a postoperative patient.58
Nuclear Medicine
Both technetium-99m (Tc-99m)–labeled bone scintigraphy and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) can detect metabolic activity in benign and malignant musculoskeletal tumors, as well as metastatic disease to the skeleton. However, these imaging techniques generally are not used to narrow the differential diagnosis of a solitary musculoskeletal tumor. Their main roles include detection of metastatic disease, staging/restaging of metastatic disease, and detection of recurrent disease. FDG-PET and bone scintigraphy are both sensitive and specific for the detection of metastatic disease from the two most common pediatric primary bone tumors: Ewing’s sarcoma and osteosarcoma. Recent studies have found FDG-PET to be more sensitive and specific for the detection of metastatic Ewing’s sarcoma; bone scintigraphy is more sensitive for the detection of metastatic osteosarcoma.15,90 FDG-PET is helpful for determining the histologic grade of musculoskeletal tumors and for assessment of nodal disease,28,32,49 and when combined with PET-CT, it has been shown to improve the accuracy of staging of musculoskeletal tumors.2
Bone scintigraphy and FDG-PET are used to detect metastatic disease and to monitor its response, with variable sensitivity and specificity depending on the disease. In patients with metastatic breast cancer, FDG-PET and bone scintigraphy have demonstrated similar sensitivities; however, FDG-PET has been shown to have higher specificity.77 Single photon emission computed tomography (SPECT) with bone scintigraphy improves lesion detection and has been shown to be more sensitive than FDG-PET for detection of breast metastases, especially those that are osteoblastic.88 18F-fluoride is a new PET agent that has demonstrated great promise for the detection of osseus metastases and was recently approved by the National Oncologic PET Registry (NOPR) for that purpose. PET-CT markedly increases the positive predictive value for bone metastases as compared with PET alone and should be used when possible.84 Whole body MRI can be used as an alternative to nuclear medicine modalities for the evaluation of metastatic disease.85
Bone Island
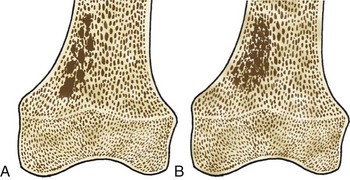
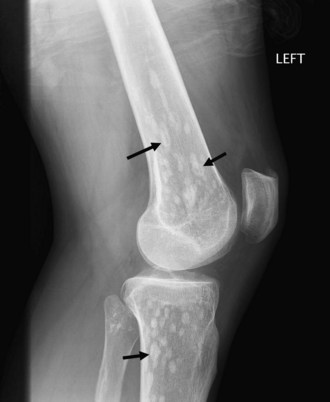
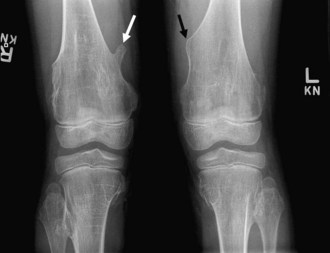
Bone islands, also known as an enostoses, are regions of compact bone located within cancellous bone. Bone islands are always benign and have a characteristic radiographic/MRI appearance. However, when large, they sometimes can be mistaken for malignant lesions such as blastic metastases. Although usually solitary, bone islands can be multiple in patients with osteopoikilosis, a benign, hereditary condition typified by multiple bone islands near joint spaces such as the knee (Fig. 11-6).
On radiography and CT, bone islands appear as sclerotic lesions with spicules that radiate peripherally. No lytic or sclerotic changes should occur in the adjacent medullary cavity, and no cortical destruction should be noted. Periosteal reaction is always absent. When bone islands are larger than 2 cm, they are called “giant bone islands.” Giant bone islands can be large, with one reported case of a 10-cm giant bone island. Although the appearance of bone islands is usually diagnostic and requires no further workup, in indeterminate cases bone scintigraphy can be performed, or follow-up radiographs/CT can be obtained at 1-, 3-, 6-, and 12-month intervals. On MRI, bone islands are extremely hypointense on T1- and T2-weighted imaging, with the degree of hypointensity the same as that of cortical bone. No marrow edema surrounds a bone island.17,53,58
Osteosarcoma
Osteosarcoma is the second most common primary bone malignancy overall and is relatively common around the knee; more than 50% occur at this location. A majority of primary intramedullary osteosarcomas occur in patients between the ages of 15 and 25, with primary intramedullary osteosarcomas rarely affecting patients younger than 6 or older than 60. Secondary osteosarcomas are much more uncommon but may occur in patients with Paget disease or following radiation therapy. Osteosarcomas most commonly involve the metaphyseal region in long bones; extension into the epiphysis is also common.65 By definition, osteosarcomas are osteoid producing; even if most of the matrix is chondroid or fibrous, when any osteoid matrix is produced by the tumor, it is characterized as an osteosarcoma by pathologists.57
A vast majority of osteosarcomas (>80%) are intramedullary, or conventional, in location and have a typical appearance. On radiography, they demonstrate a mixed lytic and sclerotic pattern, with a fluffy or osteoid matrix (Fig. 11-7). Intramedullary osteosarcomas have an aggressive radiographic appearance, wide transition zones, and periosteal reaction types such as sunburst pattern, Codman’s triangle (triangular elevation of the periosteum), or a lamellated pattern. Cortical destruction is generally present, often without cortical expansion. Because of the rapid doubling times (20 to 30 days) of these tumors, they often are very large (>6 cm) when initially discovered. Soft tissue masses accompany more than 80% of osteosarcomas, which on radiographs are seen as increased surrounding soft tissue density. CT or MRI usually is not necessary to make the diagnosis of an osteosarcoma, as the clinical history and radiographic appearance are usually diagnostic. However, in cases where the lesion is small or is atypical in appearance, either modality is helpful. Additionally, CT or MRI is necessary for staging and preoperative planning.
CT is excellent for evaluation of the internal matrix and cortical destruction; however MRI is most useful for preoperative staging and planning and is the most sensitive modality for detection of a soft tissue mass. On MRI, these tumors are hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging. The intramedullary portion also contains various quantities of elements that are hypointense on T2-weighted imaging, corresponding to the mineralized matrix. It may contain necrotic areas that are hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging, or hemorrhagic areas that are hyperintense on T1- and T2-weighted imaging. It is important to be able to detect these osteoid-producing, necrotic or hemorrhagic areas before biopsy, as these areas are to be avoided during tissue sampling. MRI demonstrates physeal involvement of the tumor and can also show involvement of the knee joint capsule. Features suggesting joint involvement include hyaline cartilage or joint capsule penetration and visualization of tumor in the suprapatellar bursa. Absence of a suprapatellar effusion means that joint involvement is unlikely.65
When staging the patient with osteosarcoma (or any suspected bone tumor), it is important to image the entire involved bone, as skip lesions can occur and indicate a prognosis similar to that of a patient with metastatic disease. Distant metastatic disease can be assessed with PET-CT or bone scintigraphy, with whole body MRI demonstrating promise for detection of multifocal osteosarcomas and other pediatric skeletal tumors.54 After treatment with chemotherapy and before surgical excision, follow-up MRI can help predict the treatment response, as greater than 90% necrosis post chemotherapy predicts an improved outcome.65 Decreased uptake on FDG-PET in osteosarcoma patients after chemotherapy has been correlated with a better outcome.9
A less common type of intramedullary osteosarcoma is the telangiectatic osteosarcoma, which is an osteoid-producing sarcoma containing large vascular channels. Sixty-two percent of telangiectatic osteosarcomas occur around the knee, with approximately three quarters of these found in the distal femur.24 As with more conventional intramedullary osteosarcomas, telangiectatic osteosarcomas are predominantly metaphyseal in location. By definition, at least 90% of a telangiectatic osteosarcoma must be composed of cystic, necrotic, or hemorrhagic areas. Radiographically, these are aggressive lytic lesions with wide transition zones, aggressive periosteal reaction, and oftentimes expansile components (Fig. 11-8). When expansile, these tumors can be confused radiographically for aneurysmal bone cysts (ABCs), and can demonstrate fluid-fluid levels on MRI. However, telangiectatic osteosarcomas have tumor cells lining the periphery and vascular channels that demonstrate nodular enhancement—a feature not seen with ABCs. CT can also be useful for demonstration of small areas of osteoid matrix—a feature that also is not seen with ABCs.24,68
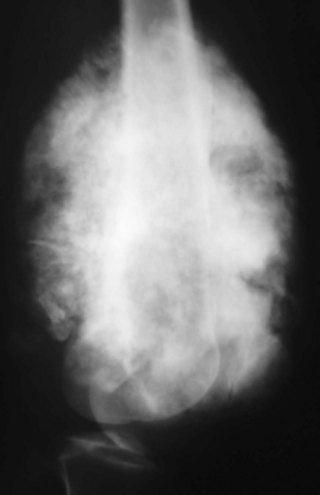
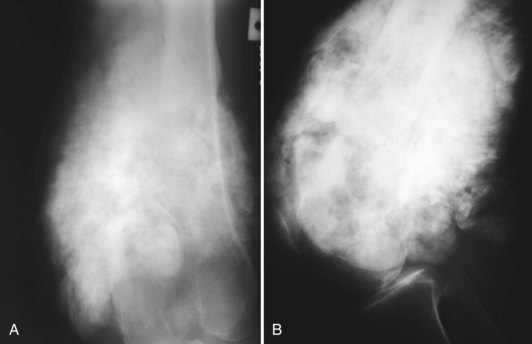
A small percentage (7% to 10%) of osteosarcomas are located on the surface of the bone.65 The two main subtypes of surface osteosarcomas are parosteal and periosteal. Parosteal osteosarcomas arise from the outer layer of the periosteum and occur most commonly in the posterior distal femoral metaphysis. Radiographically, they appear as partially ossified, exophytic lesions in soft tissues adjacent to the bone with a dense stalk connecting the lesion to the periosteum. A cleavage plane often is seen on either side of the stalk that separates the lesion from the bone, which is best visualized on CT. These tumors can demonstrate backgrowth into the medullary canal. Parosteal osteosarcomas can be mistaken for myositis ossificans, a benign bony proliferative response in the soft tissues. However, parosteal osteosarcomas demonstrate increased density centrally, whereas myositis ossificans demonstrates higher density peripherally with a lucent center (Fig. 11-9). Additionally, myositis ossificans usually is not attached to the underlying bone. On MRI, ossified portions of parosteal osteosarcomas are hypointense on all pulse sequences.
Periosteal osteosarcomas arise from the deep layer of periosteum and are three times less common than parosteal osteosarcomas. These tumors are usually metadiaphyseal in location and cause scalloping and thickening of the diaphyseal cortex, usually without involvement of the underlying medullary cavity. They demonstrate aggressive periosteal reactions and have associated soft tissue masses. Involvement of the medullary cavity can be differentiated from reactive marrow edema, as the former is contiguous with the overlying tumor. These tumors have a chondroblastic component, which on MRI is hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.23,63,65
Osteochondroma
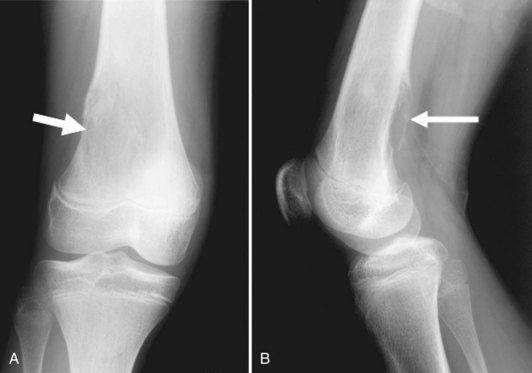
Osteochondromas are the most common benign bone tumors, with 40% occurring around the knee. Osteochondromas can be primary in isolation or in association with multiple hereditary exostoses, or they can occur secondary to prior radiation therapy.46 These benign tumors can be pedunculated or sessile, with the former significantly more common, especially in the long bones about the knee. They have a characteristic radiographic appearance, with a bony protuberance in continuity with the medullary space and the cortex pointing away from the nearest joint (Fig. 11-10). The cartilaginous cap is not well evaluated radiographically, although it sometimes can be identified by the typical “ring and arc” calcifications seen in cartilaginous lesions. CT, MRI, and even ultrasound can be used to assess the thickness of the cartilaginous cap in osteochondroma. Although one study demonstrated ultrasound to be more accurate than CT and equivalent to MRI in assessing the cartilaginous cap, MRI remains the most commonly used modality for this purpose.51 On MRI, the hyaline cartilaginous cap is identified by its hypointense signal on T1-weighted imaging and its hyperintense signal on T2-weighted imaging, lining the hypointense cortical signal present on all sequences.
Malignant transformation of a solitary osteochondroma is rare, occurring only 1% of the time, and occurring 3% to 5% of the time in patients with multiple hereditary exostoses (Fig. 11-11). Suspicion of malignant transformation should be raised in patients with pain or increased size of the osteochondroma. Radiographically, increases in size of the osteochondroma, focal lucencies in the medullary portion of the osteochondroma, changes in appearance of the cartilaginous cap, and an adjacent partially calcified soft tissue mass are all features that should raise suspicion for malignant transformation.59 On MRI in skeletally mature patients, a cap thickness greater than 1.5 cm should raise concern for a juxtacortical chondrosarcoma. One study demonstrated the average thickness of the cap in benign osteochondromas in adults to be 8 mm, with 81% of malignant lesions possessing a cap greater than 2 cm in thickness.22 Increased thickness of the cartilaginous cap in children should not raise concern for malignant transformation.59
Enchondroma
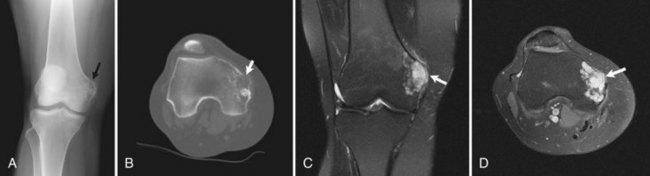
Enchondromas are relatively common around the knee and are found in 3% of routine knee MRI examinations.90 The distal femur is twice as common a site for incidental enchondromas as the proximal tibia and fibula combined. These are generally metaphyseal lesions when found about the knee, although diaphyseal enchondromas also occur.91 Solitary epiphyseal enchondromas are rare.67 Radiographically, enchondromas appear as lytic lesions with a sclerotic or nonsclerotic border, and they contain a calcified chondroid matrix that is commonly described as “ring and arc.” This matrix should be visible on radiographs; however if it is not, CT can be performed for better evaluation. These benign tumors can cause mild endosteal scalloping, although scalloping of more than two thirds of the width of the cortex, along with other criteria such as size greater than 4 cm, raises concern for chondrosarcoma.7,14,60,91 Enchondromas should never have an associated soft tissue mass or periosteal reaction. Enchondromas are often mistaken for medullary bone infarctions on radiographs because of the presence of dystrophic calcifications in the latter; however, bone infarctions usually have serpentine, peripheral calcifications and do not cause endosteal scalloping.58 CT can also help distinguish between these two entities, although often it is unnecessary. On MRI, the hyaline cartilage within enchondromas gives it hyperintense signal on T2-weighted imaging, with interspersed hypointense areas on T2-weighted imaging representing the calcified portions (Fig. 11-12). MRI also nicely depicts the lobulated contours seen in enchondromas and all hyaline cartilage neoplasms.7,60,91
Chondrosarcoma
Presence or absence of pain, location, and age are the three best factors for differentiation between enchondromas and conventional intramedullary chondrosarcomas. However, in patients presenting with internal derangement of the knee, pain may be a confounding factor that is not helpful in narrowing the differential diagnosis. As such, understanding the imaging features common to chondrosarcomas can help distinguish between the two entities. Similar to enchondromas, chondrosarcomas appear as lytic lesions with areas of mineralized matrix; however, chondrosarcomas are more likely to have certain aggressive radiographic features. As mentioned previously, chondrosarcomas commonly (75% of the time on radiographs) result in endosteal scalloping greater than two thirds of the cortical width, whereas this degree of endosteal scalloping with enchondromas is rare (9% of the time on radiographs). The degree of endosteal scalloping should be assessed at its most prominent point and has been described by Murphey et al as the most sensitive factor for differentiation between enchondromas and chondrosarcomas.60 CT or MRI should be used to assess the degree of endosteal scalloping, if it is first appreciated on radiographs. Chondrosarcomas are more likely than enchondromas to cause cortical thickening, cortical destruction, and periosteal reaction, as the tumor breaks out of the medullary space. More than half (59%) of chondrosarcomas have associated soft tissue masses, and these soft tissue masses often demonstrate low density areas and chondroid calcifications. Finally, chondrosarcomas are generally larger than enchondromas, with most chondrosarcomas greater than 4 cm in size and nearly half greater than 10 cm in length.60,75
Chondrosarcomas have features on MRI that can distinguish them from enchondromas. As with enchondromas, they have areas that are hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging, consistent with hyaline cartilage. Some foci are hypointense on all sequences, consistent with mineralized matrix. However, chondrosarcomas are more likely to produce peritumoral edema and to have septal/peripheral enhancement—two features less commonly seen with enchondromas.60
Bone scintigraphy can be used to differentiate between enchondromas and chondrosarcomas. One study demonstrated that 82% of chondrosarcomas had uptake greater than that of the anterior iliac crest, as opposed to only 21% of enchondromas, when conventional bone scintigraphy was used.60 Another study using Tc-99m–labeled dimercaptosuccinic acid (DMSA) found that all cases of chondrosarcoma that had already showed increased uptake with Tc-99m methyl diphosphonate (MDP), had increased activity with Tc-99m DMSA.36 More recent studies have focused on FDG-PET as a modality for distinguishing between benign and malignant cartilaginous neoplasms.12
Clear cell chondrosarcomas are rare chondrosarcomas found most commonly in the epiphyses of long bones. These tumors most commonly appear as geographic 1A or 1B lesions and contain chondroid matrix and for these reasons and their typical location can be mistaken for chondroblastomas. Although making the imaging distinction between the two entities can be difficult, factors that make clear cell chondrosarcomas more likely include larger size, signal that is more hyperintense on T2-weighted imaging, and increased enhancement relative to that seen in chondroblastomas. Additionally, clear cell chondrosarcomas are most common in the femoral head rather than in the distal femur, and they typically occur in patients a decade older than those with chondroblastoma. Finally, clear cell chondrosarcomas are much more common in males8,58 (Fig. 11-13).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree