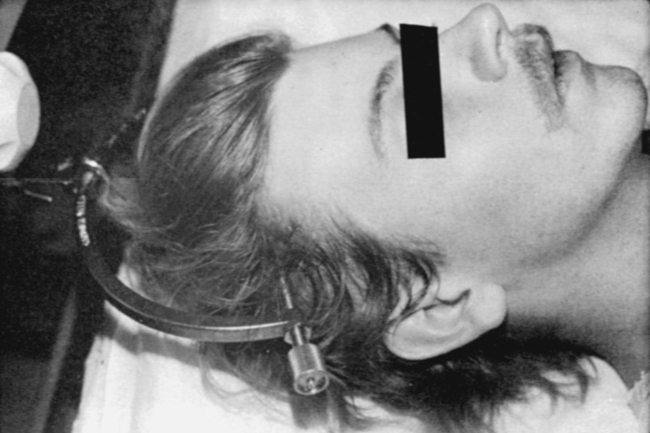
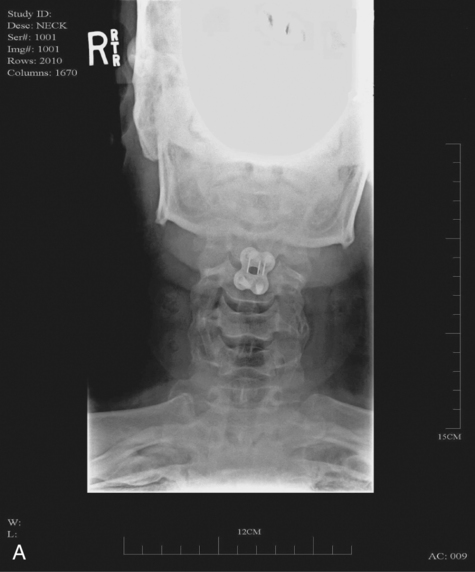
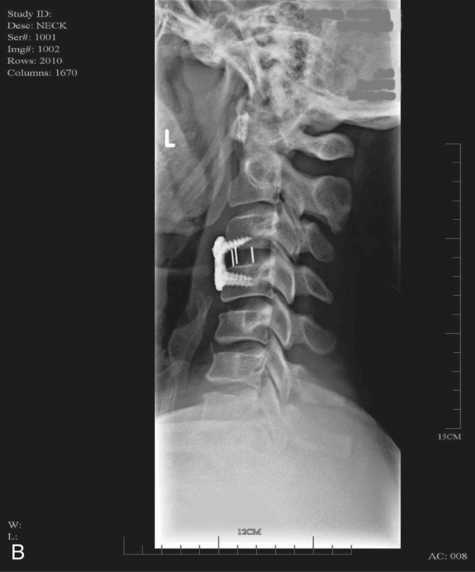
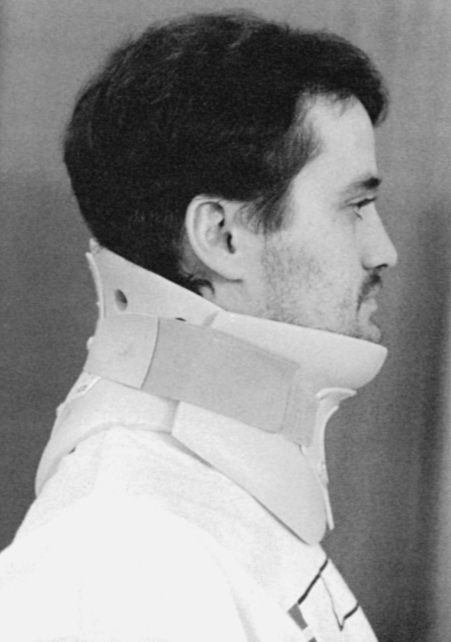
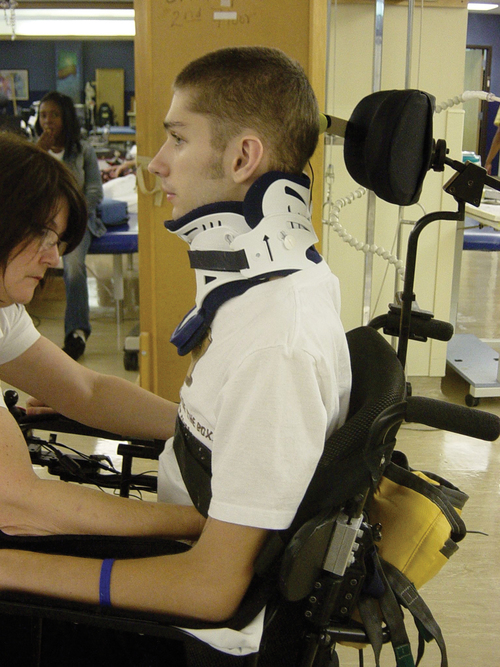
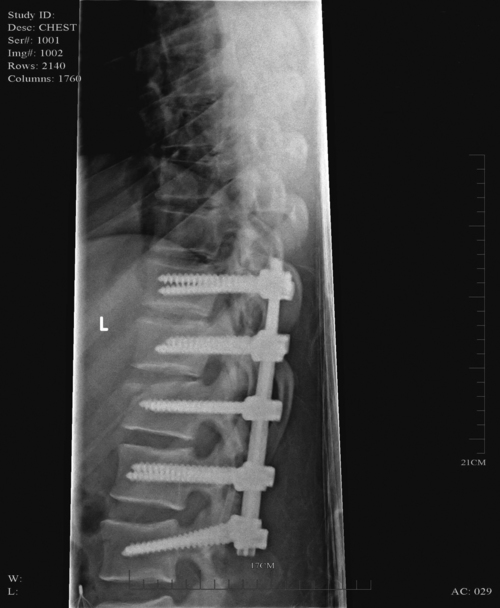
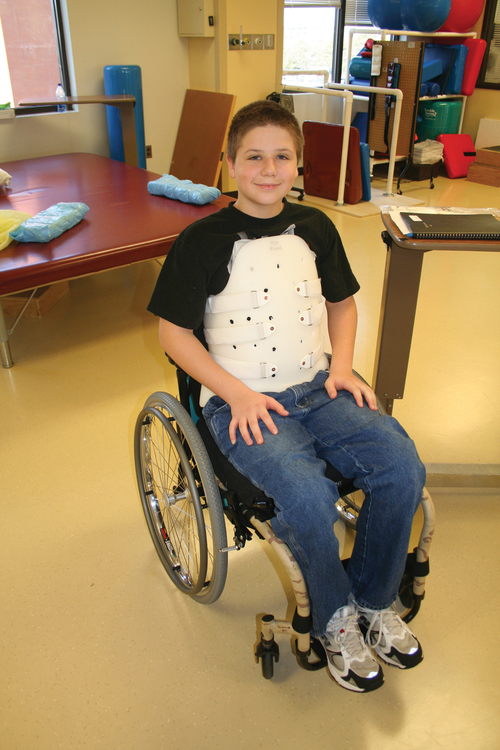
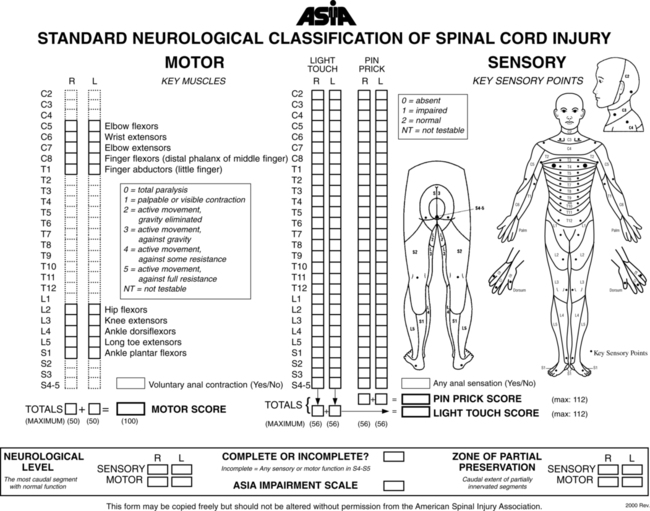
MYRTICE B. ATRICE, PT, BS, SARAH A. MORRISON, PT, BS, SHARI L. McDOWELL, PT, BS, PAULA M. ACKERMAN, MS, OTR/L, TERESA A. FOY, OT, BS and CANDY TEFERTILLER, DPT, ATP, NCS After reading this chapter the student or therapist will be able to: 1. Describe the demographics, etiology, and mechanism of injury of spinal cord injury. 2. Discuss the acute medical management of person with spinal cord injury. 3. Describe the secondary complications of spinal cord injury, the appropriate interventions, and the impact of complications on the rehabilitation process. 4. Identify the basic components of the examination process. 5. Identify patient problems based on the examination, establish appropriate goals, and plan individualized treatment programs for patients with a spinal cord injury. 6. Describe adaptive equipment available to increase function. 7. Discuss progression of each individual and the process of discharge planning throughout the rehabilitation process. 8. Describe functional expectations for individuals with complete spinal cord injuries. 9. Identify equipment needs for a given spinal cord injury lesion. 10. Describe various aspects of activity-based therapies to promote recovery after spinal cord injury. Spinal cord injury (SCI) is a catastrophic condition that, depending on its severity, may cause dramatic changes in a person’s life. SCI usually happens to active, independent people who at one moment are in control of their lives and in the next moment are paralyzed, with loss of sensation and loss of bodily functions, which can lead to dependence on others for even the most basic needs. To reduce negative impact, individuals with SCI need a well-coordinated, specialized rehabilitation program to assist them in maximizing the development of skills necessary to live a satisfying and productive postinjury life.1,2 A successful rehabilitation program requires a team of health care professionals who work in unison to address alterations in body function, increase the individual’s independence in all daily activities, and return the individual to the highest level of community participation specific to that individual’s life situations. Minimally, the team should include a physician, case manager, occupational therapist, physical therapist, therapeutic recreation specialist, prosthetist or orthotist, nurse, speech-language pathologist, dietician, assistive technologist, respiratory care practitioner, psychologist, social worker, vocational counselor, rehabilitation engineer, and chaplain.3–5 The most important element determining success in any rehabilitation program is the patient’s and family’s active participation throughout the rehabilitation process. Tetraplegia (preferred to quadriplegia) refers to impairment or loss of motor and/or sensory function as a result of damage to the cervical segments of the spinal cord. Function in the upper extremities, lower extremities, and trunk is affected. It does not include brachial plexus lesions or injury to peripheral nerves outside the neural canal.6 Paraplegia refers to impairment or loss of motor or sensory function as a result of damage to the thoracic, lumbar, or sacral segments of the spinal cord. Depending on the level of the damage, function may be impaired in the trunk and/or lower extremities. This term is used to refer to cauda equina and conus medullaris injuries but not to lumbosacral plexus lesions or injury to peripheral nerves, which are considered outside of the central nervous system.6 In a complete lesion, sensory and motor function in the lowest sacral segments (S4-S5) is absent postinjury.6 The American Spinal Injury Association (ASIA) classification for this type of injury is ASIA Impairment Scale (AIS) A. Complete injuries to the spinal cord are usually the result of extensive trauma or disease and are often segmentally associated with damage to the nerve roots in the intervertebral foramina.7 Function of the roots originating from the more cranial portion of the intact cord can be expected to return within 6 months.7 Discomplete injury is a relatively new term in SCI research and practice. It is defined as a lesion that is “clinically complete but which is accompanied by neurophysiological evidence of residual brain influence on spinal cord function below the level of the lesion.”8 Studies of persons whose spinal cord injuries were considered complete under ASIA standards have shown that in a large percentage (84%) there was residual brain influence on the spinal cord below the level of the lesion.8,9 The current gold standard for testing, the AIS, is unable to detect this residual function, which suggests that AIS testing may be providing an inaccurate picture of the patients’ neurological plasticity and recovery potential. The Brain Motor Control Assessment (BMCA) is emerging as a desirable adjunct to the standard ASIA testing.9 In the BMCA, surface electromyography (EMG) is used to quantify the motor unit activity of the lower extremities in response to a standard testing protocol including active and passive movement of the lower extremities, reinforcement maneuvers (e.g., Jendrassik or Valsalva) performed above the level of injury, tendon taps and vibration, and elicitation and suppression of reflex activity. The motor unit responses are quantified and compared with normative data to establish a voluntary response index and a similarity index. In other words, the results of the BMCA help to determine how different the subjects’ motor responses are from those of persons with intact neurological systems.8–11 This testing requires specialty equipment but can easily be administered by physical therapists once they have received the appropriate training. With incomplete lesions there is detectable residual sensory or motor function below the neurological level and specifically in the lowest sacral segment. According to ASIA standards, any sensation in the anal mucocutaneous junction, or deep anal sensation, indicates that the lesion is incomplete. If only sensation is preserved, the injury is classified as AIS B. If motor function in key muscles is maintained to some degree, patients may achieve level C, D, or E classification. This testing will be reviewed further in this chapter.6,12 The incidence of traumatic SCI in the United States is approximately 12,000 new cases per year.13 Approximately 3000 new cases of spinal cord impairment resulting from disease and congenital anomalies occur each year. The number of people living in the United States today with SCI is between 231,000 and 311,000.13 Fifty-three percent of traumatic SCIs occur in persons aged 16 to 30 years. However, the median age of the general population of the United States has increased by 8 years since the mid 1970s, and the average age of the SCI population has steadily increased. Since 2005, the mean age at the time of injury is 40.2 years.13,14 Persons older than 60 years of age at injury have increased from 4.7% before 1980 to 11.5% for injuries occurring since 2000. This trend explains the increase in the median age during this same time period from 27.9 years to 35.3 years. Table 16-1 lists additional demographics. TABLE 16-1 SPINAL CORD INJURY DEMOGRAPHICS Data from National Spinal Cord Injury Statistical Center: Spinal cord injury: facts and figures at a glance, February 2010, Birmingham, AL, 2010, University of Alabama, National Spinal Cord Injury Statistical Center. Available at www.uab.edu/NSCICSC. In 2005 the average length of inpatient stay was 50 days (12 days in an acute-care facility and 38 days in rehabilitation). The average yearly health care and living expenses vary according to severity of injury. In the first year, individuals with high tetraplegia spend $829,843, whereas individuals with paraplegia spend an average of $303,220.13 Today 87.7% of persons with SCI are discharged to a noninstitutional residence. Life expectancies for patients with SCI continue to increase but are still below the national average of persons without SCI. Mortality rates are significantly higher during the first year after injury, especially for severely injured persons. According to the National SCI Database, the leading causes of death after an SCI are pneumonia, pulmonary emboli, and septicemia.13 Statistics suggest a high incidence of multiple trauma associated with a traumatic SCI (55.2%).15 The most common injuries are fractures (29.3%) and loss of consciousness (28.2%).15 Traumatic pneumothorax or hemothorax are reported in 17.8% of persons with SCI. Traumatic head injuries of sufficient severity to affect cognitive or emotional functioning are reported in 11.5% of all cases.15 Skull and facial fractures, along with traumatic head injuries and vertebral artery and esophageal disruptions, are common in cervical injuries.16 Limb fractures and intrathoracic injuries (rib fractures and hemopneumothorax) are frequent in thoracic injuries, whereas intraabdominal injuries to the liver, spleen, and kidneys are associated with lumbar and cauda equina injuries.16 As stated previously, most spinal cord injuries occur as a result of trauma, be it motor vehicle accidents, falls, violence, or sports-related injury. The degree and type of forces that are exerted on the spine at the time of the trauma determine the location and severity of damage to the spinal cord.17 Injuries to the vertebral column can be classified biomechanically as flexion or flexion-rotation injuries, hyperextension injuries, and compression injuries.18 Penetrating injuries to the cord are usually the result of gunshot or knife wounds.18 Spinal cord damage can also be caused by nontraumatic mechanisms. Circulatory compromise to the spinal cord resulting in ischemia causes neurological damage at and below the involved cord level. This can be caused by a thrombus, swelling, compression, or vascular malformations and dysfunction. Degenerative bone diseases can cause compression of the spinal cord by creating a stenosis of the spinal canal and intervertebral foramina. Stenosis can also result from the prolapse of the intervertebral disc into the neural canal. The encroachment of tumors or abscesses within the spinal cord, the spinal canal, or the surrounding tissues can also lead to SCI. Congenital malformation of the spinal structures, as in spina bifida, can also compromise the spinal cord and its protective layers of connective tissue. Some of the more common diseases and conditions that result in compromise of the spinal cord include Guillain-Barré syndrome, transverse myelitis, amyotrophic lateral sclerosis, and multiple sclerosis.12 After the spinal cord has sustained damage, cellular events occur in response to the injury and are classified in three phases of progression: acute, secondary, and chronic responses. The acute process begins on occurrence of an injury and continues for 3 to 5 days.19 Abrupt necrosis or cell death can result from both mechanical and ischemic events. The impact of an SCI often causes direct mechanical damage to neural and other soft tissues as well as severe hemorrhaging in the surrounding gray and white matter, resulting in immediate cell death.20,21 In the next few minutes after the insult, injured nerve cells respond with trauma-induced action potentials, which lead to increased levels of intracellular sodium. The result of this influx is an increase in osmotic pressure movement of water into the area. Edema generally develops in up to three levels above and below the original insult and leads to further tissue deconstruction.19,21,22 Increased levels of extracellular potassium and intracellular concentrations of calcium also result in an electrolyte imbalance that contributes to a toxic environment.23–25 Abnormal concentrations of calcium within the damaged cells disrupt their functioning and cause breakdown of protein and phospholipids, leading to demyelination and destruction of the cell membrane.25 The cascade of these events consequentially contributes to a dysfunctional nervous system. During this acute phase, evidence of spinal shock may be present. Spinal shock occurs 30 to 60 minutes after spinal trauma and is characterized by flaccid paralysis and absence of all spinal cord reflex activity below the level of the spinal cord lesion.26,27 This condition lasts for about 24 hours after injury, represents a generalized failure of circuitry in the spinal neural network, and is thought to be directly related to a conduction block resulting from leakage of potassium into the extracellular matrix.28 The completeness of the lesion cannot be determined until spinal shock is resolved. The signs of spinal shock resolution are controversial; however, the return of reflexes may be a good indication. The secondary phase of the injury occurs within the course of minutes to weeks after the acute process and is characterized by the continuation of ischemic cellular death, electrolytic shifts, and edema. Extracellular concentrations of glutamate and other excitatory amino acids reach concentrations that are six to eight times greater than normal within the first 15 minutes after an injury.24 In addition, lipid peroxidation and free radical production also occur.29 Apoptosis (a secondary programmable cell death) occurs and involves reactive gliosis. There is also an important immune response that adds to the secondary damage that may be a result of a damaged blood-brain barrier, microglial activation, and increased local concentrations of cytokines and chemokines.30 The lesion enlarges from the initial core of cell death, expanding from the perilesional region to a larger region of cell loss. In the chronic phase, which occurs over a period of days to years, apoptosis continues both rostrally and caudally. Receptors and ion channels are altered, and with penetrating injuries scarring and tethering of the cord occurs. Conduction deficits persist owing to demyelination, and permanent hyperexcitability develops with consequential chronic pain syndromes and spasticity in many SCI patients.26 Changes in neural circuits result from alterations in excitatory and inhibitory inputs, and axons may exhibit regenerative and sprouting responses but go no farther than 1 mm.24 Medical interventions are evolving to limit the impact of the acute SCI and the subsequent progression that follows. Growing interest in protection and repair of the injured nervous system has led to an improved understanding of the pathophysiology associated with SCI and has resulted in the implementation of several therapeutic strategies that are currently being investigated in phase 1 and 2 clinical trials. The effects of methylprednisolone sodium succinate, tirilizad mesylate, monosialotetrahexosylganglioside, thyrotropin-releasing hormone, gacyclidine, naloxone, and nimodipine have all been examined in randomized controlled trials over the last few years. Although the primary outcomes in these studies did not demonstrate statistically significant effects, a secondary analysis demonstrated that methylprednisolone sodium succinate given within 8 hours of injury was associated with modest clinical benefits.17 Phase 2 trials with monosialotetrahexosylganglioside and thyrotropin-releasing hormone also yielded some therapeutic benefits, but further studies need to be completed to determine efficacy. Several current or planned studies exist to evaluate the potential benefits of early surgical decompression and electrical field stimulation, neuroprotective strategies such as riluzole and minocycline, the inactivation of myelin inhibition by blocking Nogo and Rho, and the transplantation of various substrates into the injured spinal cord.17 Promising clinical trials are also underway to minimize the secondary phase of injury and to promote healing and neuronal regeneration (Table 16-2). If medical interventions can bridge the central lesion or limit the secondary progression, the functional loss that follows SCI will be minimized and chances of recovery improved. TABLE 16-2 Data from Hulsebosch CE: Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ 26:238–255, 2002; and Sekhon LH, Fehlings MG: Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 26(24 suppl):S2–S12, 2001. Some incomplete lesions have a distinct clinical picture with specific signs and symptoms. An understanding of the various syndromes can be helpful to the patient’s team in planning the rehabilitation program. Figure 16-1 depicts the anatomy of the spinal cord.30,31 This basic anatomy of the spinal cord can be referred to as the various syndromes are described. Hyperextension injuries usually result in a central cord syndrome.31 This injury causes bleeding into the central gray matter of the spinal cord, resulting in more impairment of function in the upper extremities than in the lower extremities.31 Most incomplete lesions result in this syndrome, especially in elderly individuals when cervical stenosis is present.30 Although the prognosis for functional recovery is good for individuals with central cord syndrome, the pattern of recovery is such that intrinsic hand function is the last thing to return. Approximately 77% of clients with central cord syndrome will attain some level of ambulatory function, 53% bowel and bladder control, and 42% hand function.12,32,33 Anterior spinal artery syndrome is usually caused by flexion injuries in which bone or cartilage spicules compromise the anterior spinal artery.31 Motor function and pain and temperature sensation are lost bilaterally below the injured segment.31 The prognosis is extremely poor for return of bowel and bladder function, hand function, and ambulation.12,33 Occasionally, as a result of penetrating injuries (gunshot or stab wounds), only one half of the spinal cord is damaged. The Brown-Séquard syndrome is characterized by ipsilateral loss of motor function and position sense and contralateral loss of pain sensation several levels below the lesion.31 The prognosis for recovery is good. Nearly all clients attain some level of ambulatory function, 80% regain hand function, 100% have bladder control, and 80% have bowel control.12,33 One of the first interventions after acute traumatic SCI is to stabilize the spine to prevent further cord or nerve root damage. In the emergency department, diagnostic studies reveal the severity of the spinal injury and the type and degree of the instability. On the basis of these findings, the physician, client, and family decide on treatment. Many options must be considered regarding the optimal operative strategy. Indications for surgical intervention include, but are not limited to, signs of progressive neurological involvement, type and extent of bony lesions, and degree of spinal cord damage.34 The following discussion describes nonsurgical and surgical interventions. At the scene of the accident, emergency medical professionals exercise extreme caution to immobilize the injured patient and prevent excessive movement. If there is compression of neurological tissue, vertebral fracture, or dislocation, reduction must occur to minimize ischemia and edema formation.35 In the emergency department, reduction is accomplished by cervical traction with the goal of immediate and proper alignment of bone fragments and decompression of the spinal cord until further stabilization.34,36,37 The most widely used traction method is the Gardner-Wells tongs (Figure 16-2), which are inserted into the skull. Weights are added at approximately 5 pounds of traction per level of injury to achieve reduction of the dislocation and to maintain alignment.36 When surgical stabilization is indicated, common surgical protocols include posterior and anterior approaches. Figure 16-3 shows radiographs of a person who had an anterior and lateral cervical fusion at C3-C4. Unstable compression injuries are usually managed by a posterior procedure except when there is a deficient anterior column. Anterior approaches are indicated for patients with evidence of residual anterior spinal cord or nerve root compression and persistent neurological deficits.34 After cervical surgical stabilization, a hard collar such as a Philadelphia collar (Figure 16-4) or sternal-occipital-mandibular immobilizer (SOMI) brace is used until solid bony fusion has developed. The Aspen collar also provides this stability (Figure 16-5). The solid bony fusion usually takes 6 to 8 weeks. Postoperatively, care must be taken to protect the bony fusion. When surgery is not indicated, or when more postoperative stabilization is required, halo traction may be indicated. The halo device restricts more movement in the upper cervical spine compared with the lower cervical spine.38 The halo traction device consists of three parts: the ring, the uprights, and the jacket (Figure 16-6). The ring fits around the skull, just above the ears. It is held in place by four pins that are inserted into the skull. The uprights are attached to the ring and jacket by bolts. The jacket is usually made of polypropylene and lined with sheepskin. This equipment is left in place for 6 to 12 weeks until bony healing is satisfactory.5 The advantage of using the halo device is the ability to mobilize the client as soon as the device has been applied without compromising spinal alignment. This allows the rehabilitation program to commence more rapidly. It also allows for delayed decision making regarding the need for surgery. The disadvantage of the halo device is that pressure and friction from the vest or jacket may lead to altered skin integrity.7 Special attention must be given to ensure the skin remains intact. During more active phases of the rehabilitation process, the halo device may slow functional progress because of added weight and interference with the middle to end range of upper-extremity movement. In a small percentage of patients, there are complications of dysphagia and temporomandibular joint dysfunctions associated with wearing the halo device.7 Internal fixation of the thoracolumbar region is necessary when stability and distraction cannot be maintained by other means.39 Common thoracic stabilization procedures include transpedicular screws (Figure 16-7) and a hybrid type of instrumentation. Postoperatively, an external trunk support may be necessary to limit excessive vertebral motion and to maintain proper thoracic and lumbar alignment.39 This may be achieved by a custom thoracolumbosacral orthosis (Figure 16-8) or a Jewett brace (Figure 16-9). Initially the client’s activity may be limited to allow for a complete fusion to take place and to minimize the possibility of rod displacement. All spinal limitations should be discussed with the surgeon postoperatively. The goals of the operative procedures at any spinal level discussed are to reverse the deforming forces, to restore proper spinal alignment, and to stabilize the spine.40 All these procedures have advantages and disadvantages. The surgeon, client, and family must be involved in the decision-making process to select the most appropriate method of treatment. This will allow the therapeutic rehabilitation process to begin. The National Acute Spinal Cord Injury Study41 (NASCIS-2) used high doses of methylprednisolone and showed significant improvements in sensory and motor function 6 months after injury.42 Young and Flamm43 showed that methylprednisolone enhanced the flow of blood to the injured spinal cord, preventing the typical decline in white matter, extracellular calcium levels, and evoked potentials, thus preventing progressive posttraumatic ischemia.44–47 The dosage recommended by the NASCIS-2 study is 30 mg/kg of methylprednisolone followed by an infusion of 5.4 mg/kg/hr for 23 hours.41 The therapist must be aware of side effects that may occur with such high doses of steroids, including gastric ulcers, decreased wound-healing time, hypertension, cardiac arrhythmias, and alteration in mental status.42 Therapeutic rehabilitation can be effectively delivered beginning in an acute-care setting at the time of injury and continuing on through a lifetime of care. Rehabilitation teams may use one of three models: multidisciplinary, interdisciplinary, and transdisciplinary.3 The standards set forth by the Commission on Accreditation of Rehabilitation Facilities (CARF) suggest that the interdisciplinary model of team structure is optimal in the rehabilitation setting.31 As the acute phase progresses, out-of-bed activities are tolerated for longer periods of time and the patient begins to work toward specific long-term goals. In accordance with Medicare guidelines for inpatient rehabilitation, the client is able to participate in therapeutic programs a minimum of 3 hours a day.48,49 The intensity of therapy may continue to be limited according to unresolved medical issues. Discharge from an inpatient rehabilitation program marks only the beginning of the lifelong process of adjustment to changes in physical abilities, community reintegration, and participation in life activities. Inpatient rehabilitation provides an environment best suited for learning self-care skills, yet “the implications of living in the community with SCI can scarcely be anticipated accurately by the newly injured individual or the able-bodied staff.”50 Because of the shortened lengths of hospitalization, services provided after discharge are becoming increasingly important. A direct consequence of this shift results in outpatient treatment of patients who have more acuity, greater care needs, and fewer skills attained in the inpatient rehabilitation program before entry into the outpatient arena. Common outpatient therapy treatment programs have included advanced transfer training, advanced wheelchair mobility training, locomotor training, upgraded ADL training, and upgraded home exercise program instruction. This is a shift in the typical program structure because these skills were traditionally a part of the inpatient rehabilitation. Regardless of where the patient begins the rehabilitation process, an examination is completed on admission. The examination and evaluation will assist in establishing the diagnosis and the prognosis of each patient as well as determining the appropriate therapeutic interventions. The client and caregivers participate by reporting activity performance and functional ability.51 Any pertinent additions to the history stated by the client should be described. The client’s statement of goals, problems, and concerns should be included. The main areas of the examination are outlined here. A review of the medical record is the first step toward the examination because it provides the background information and identifies medical precautions. The history should include general demographics, social history, occupation or employment, pertinent growth and development, living environment, history of current condition, functional status and activity level, completed tests and measures, medications, history of current condition if applicable, medical and surgical history, family history, reported patient and family health status, and social habits.52 If the history suggests a loss of consciousness or brain injury, the clinician should consider the possibility of compromised cognition and should include tests and measures during the examination and assessment appropriate to that impairment. Depending on the data generated during the history and systems review, the clinician performs tests and measures to help identify impairments, activity limitations, and participation restrictions and to establish the diagnosis and prognosis of each client. Tests and measures that are often used for persons with SCI are included in Box 16-1. For more detail related to specific tools, refer to the Guide to Physical Therapist Practice.52 It is recommended that the international standards of ASIA be used for the specific neurological examination after an SCI.53 See Figure 16-10 for the ASIA motor and sensory examination form. Assessment of muscle performance allows for specific diagnosis of the level and completeness of injury. The examination of muscle performance includes each specific muscle and identifies substitutions from other muscles.
Traumatic spinal cord injury
Spinal cord lesions
Tetraplegia
Paraplegia
Complete, discomplete, and incomplete lesions
Demographics

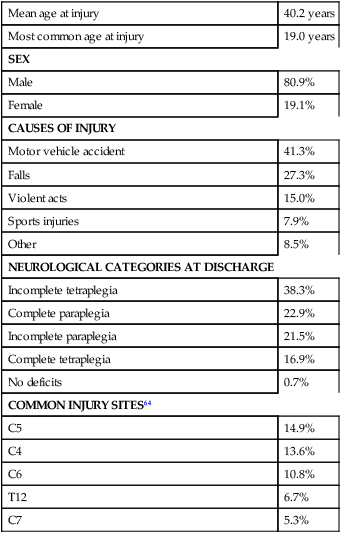
Mean age at injury
40.2 years
Most common age at injury
19.0 years
SEX
Male
80.9%
Female
19.1%
CAUSES OF INJURY
Motor vehicle accident
41.3%
Falls
27.3%
Violent acts
15.0%
Sports injuries
7.9%
Other
8.5%
NEUROLOGICAL CATEGORIES AT DISCHARGE
Incomplete tetraplegia
38.3%
Complete paraplegia
22.9%
Incomplete paraplegia
21.5%
Complete tetraplegia
16.9%
No deficits
0.7%
COMMON INJURY SITES64
C5
14.9%
C4
13.6%
C6
10.8%
T12
6.7%
C7
5.3%
Sequelae of traumatic spinal cord injury

PHASE
DESCRIPTION
Acute
Systemic hypotension and spinal shockHemorrhage
Cell death from direct insult or ischemia
Edema
Vasospasm
Shifts in electrolytes
Accumulation of neurotransmitters
Induced hypothermic treatment
Secondary
Continued cell deathContinued edema
Continued shifts in electrolytes
Free-radical production
Lipid peroxidation
Neutrophil and lymphocyte invasion and release of cytokines
Apoptosis
Calcium entry into cells
Chronic
Continued apoptosis radiating from site of injuryAlteration of ion channels and receptors
Formation of fluid-filled cavity
Scarring of spinal cord by glial cells
Demyelination
Regenerative processes, including sprouting by neurons
Altered neurocircuits
Syringomyelia
Clinical syndromes
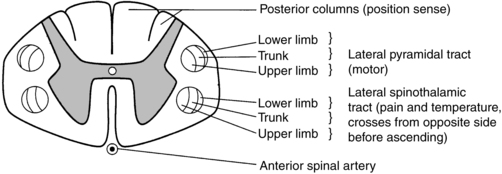
 Cross-sectional anatomy of the spinal cord.
Cross-sectional anatomy of the spinal cord.
Central cord syndrome
Anterior spinal artery syndrome
Brown-séquard syndrome
Medical management
Surgical stabilization
Cervical spine
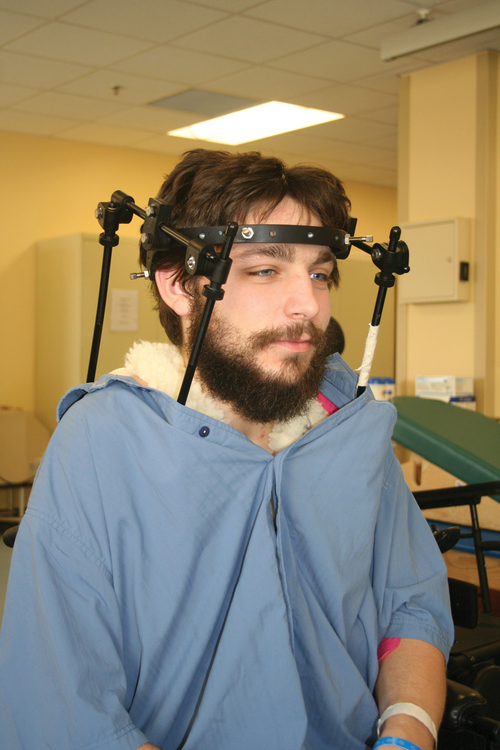
 Halo vest. Basic components are the halo ring, distraction rods, and jacket (jacket not pictured).
Halo vest. Basic components are the halo ring, distraction rods, and jacket (jacket not pictured).
Thoracolumbar spine
Pharmacological management immediately after traumatic spinal cord injury
Therapeutic rehabilitation continuum of care
Inpatient rehabilitation
Outpatient rehabilitation and community reentry
Examination and evaluation of body function and structure
History
Tests and measures
Neurological examination
American spinal cord injury association examination
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Traumatic spinal cord injury
Only gold members can continue reading. Log In or Register to continue