ALAIN CLAUDEL, PT, DPT, ECS, ROLANDO T. LAZARO, PT, PhD, DPT, GCS, GEORGE WOLFE, PT, PhD and JANET MARIE ADAMS, PT, MS, DPT After reading this chapter the student or therapist will be able to: 1. Identify electrophysiological tests performed on clients with neurological disorders. 2. Describe the instrumentation and general procedures for electrophysiological testing. 3. Recognize normal and abnormal findings of various electrophysiological tests. 4. Recognize the differences in instrumentation, signal processing, and interpretation when performing electrophysiological testing versus kinesiological electromyographic testing. 5. Differentiate the basic mechanism underlying functional neuromuscular stimulation, electrical stimulation, and electromyographic biofeedback. 6. Describe the appropriate instrumentation, signal processing, and interpretation for kinesiological electromyographic testing. 7. Describe the indications and contraindication for the use of neuromuscular stimulation, electrical stimulation, and electromyographic biofeedback. Electrophysiological tests are usually performed by neurologists, physiatrists, and PTs who have education, training, and experience in these procedures. Most PTs practicing in the area of clinical electrophysiology are board certified by the American Board of Physical Therapy Specialties.1 Some states (such as California) require additional licensing. The general goal of electrophysiological testing is to answer the following questions: The electrophysiological tests most commonly used are motor and sensory NCSs, including F-wave and H-reflex latency measurements; repetitive stimulation; somatosensory evoked potential (SSEP) tests; and needle EMG. Most often, a patient referred for electrophysiological testing will undergo at least two motor nerve conduction tests, at least two sensory conduction tests, and at least one limb needle EMG. The American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) has published evidence-based guidelines, which may be found on the AANEM website at www.aanem.org/Practice/Practice-Guidelines.aspx.2 A review of the client’s history, a relevant systems review, and a physical examination guide the examiner in the selection and sequencing of appropriate tests. In other words, muscle strength and tone, sensation, range of motion (ROM), neurological signs, and cognition are crucial in selecting and administering electrophysiological tests. Electrophysiological testing is considered by all authors on the subject as an extension of the clinical examination.3,4 It does not replace a careful history and physical examination of the patient. It does, however, establish the precise state of the nerves and muscles and can thus determine the location of a lesion more precisely than the clinical examination alone, particularly in cases of mild weakness or ill-defined sensory changes. In very clearly defined pathologies, electrophysiological tests are not necessary (except perhaps for medicolegal reasons). For example, in the case of a unilateral ankle dorsiflexion weakness coupled with a clearly defined L5 nerve root compression on magnetic resonance imaging (MRI) of the lumbar spine, the electrophysiological test may be of little added value. However, for a similar clinical presentation (foot drop) and no clear-cut imaging, the electrophysiological tests will differentiate between a peroneal palsy, a sciatic nerve neuropathy, a lumbosacral plexopathy, or an L5 radiculopathy. Finally, evidence-based practice recommends that the practitioner have a good understanding of the implication of the sensitivity and specificity of each test to rule it in or out for a specific condition.5 A nerve is composed of axons covered with a sheath of myelin. Depolarization inside the axon is an “all-or-nothing” phenomenon in which an action potential moves along the surface of the cell membrane. This action potential is an electrical wave caused by a flow of ions across the cell membrane. A local current opens a sodium channel, allowing Na+ ions to rush inside the cell. The electrical resistance to this wave is inversely proportional to the diameter of the axon. Larger nerves conduct faster than smaller nerves. In order for efficiency as an organism to be achieved, nerve conduction must be fast. In complex organisms with billions of axons, increasing the nerve diameter is not a viable option; hence, the role of the myelin sheath. The myelin is produced by Schwann cells. These are special satellite cells that separate axons from the endoneural fluid. The myelin acts as a capacitor: the conduction “jumps” between gaps in the myelin called nodes of Ranvier. This saltatory conduction allows human nerves to be 50 times smaller but conduct four times faster than unmyelinated nerves.6 Consequently, recording and analyzing the conduction velocity of nerves primarily reflects on the state of the myelin. The amplitude of the response (if a supramaximal stimulation is delivered) is a reflection of the number of axons available to the stimulation. The physiology of the nerve is such that when there is an injury to the axon, the portion of the axon distal to the injury will degenerate (wallerian degeneration). This is important because all muscles innervated by branches of the nerve distal to the lesion will show signs of denervation approximately 11 days after the lesion. Consequently, assessing a patient too early after a lesion may lead to false-negative results.6,7 The accurate performance and interpretation of the electrophysiological test—particularly the needle EMG—is significantly contingent on knowledge of the precise innervation of each muscle. As an example, an ulnar neuropathy at the elbow (UNE) clinically may be indistinguishable from a C8 radiculopathy. However, the astute clinician will remember that the cell body of the sensory nerve lies in the dorsal root ganglion, which is typically not involved in a radiculopathy. The therapist will also know that the abductor pollicis brevis is a C8- and median nerve–innervated muscle. Consequently, an ulnar nerve neuropathy is distinguishable from a C8 radiculopathy in that the ulnar sensory test will have decreased amplitude in the UNE and the abductor pollicis brevis will be denervated in the C8 radiculopathy. As a matter of fact, the clinician will keep in mind the innervation of each muscle while conducting the test. Electrophysiological testing is hence a dynamic process during which the choice of the next nerve to test or muscle to sample is predicated on the result of the previous test.6,7 As a result, the electrophysiological examination is not a single, stereotyped investigation but an evolutionary one during which several tests (nerve conduction, both sensory and motor, and EMG of several muscles) can be applied to a clinical presentation.3,8 A general overview of NCSs is presented to provide an understanding of their application and indications. Many excellent texts are available for details of the techniques.9–13 Nerve conduction velocity is faster in myelinated fibers because of saltatory conduction. Disorders involving peripheral demyelination can thus be differentiated from impairments primarily involving axonal degeneration. A mild localized compressive disorder (neurapraxia) may be distinguished from a more severe lesion in which the axons and surrounding connective tissue have been completely disrupted (neurotmesis).11,14 In the event that the findings of NCS and EMG are normal, the clinician may be able to rule out most conditions involving the PNS and look for central nervous system (CNS) or other pathology. Knowledge of the rationale for NCSs and EMG should help the therapist decide when the tests may be indicated and understand the reasoning behind reports of tests that have already been performed on clients. In motor NCSs the peripheral nerve is stimulated at various sites and the evoked electrical response is recorded from a distal muscle supplied by the nerve (a measure of orthodromic conduction). Surface electrodes are usually used for both stimulating and recording. An example of electrode configuration for a motor NCS is shown in Figure 33-1 (ulnar nerve study). The response represents the electrical activity of muscle fibers under the recording electrodes and is called the compound muscle action potential (CMAP). It is also called the M wave or M response. Measurements are taken of the latency (the time in milliseconds required for the impulse to travel from each stimulus site to the recording site) and the amplitude of the response in millivolts (mV). The shape and duration of the response are assessed, and motor nerve conduction velocity is calculated for each segment of interest by dividing the distance between stimulus sites (in millimeters) by the difference in latency measured at each respective site. Velocities, latencies, and the shape and amplitude of the responses (Figure 33-2) are studied and compared with established normal values and often with values taken from tests of the uninvolved extremity (when possible). In infants and children, nerve conduction is slower than in adults and reaches adult values by age 4 years.13 Nerve conduction velocities gradually slow after age 60 years but generally remain within the outer limits of normal.11,13 Sensory nerve conduction can be measured from many superficial sensory nerves, such as the superficial radial and sural nerves. It can also be measured from mixed motor and sensory nerves. The stimulus is applied over the nerve in question, and the recordings taken from electrodes placed over a distal sensory branch of the nerve. The recordings are called sensory nerve action potentials (SNAPs). An example of recording and stimulation sites is shown in Figure 33-3. Both orthodromic and antidromic conduction can be assessed. Response latencies and amplitudes are measured, and sensory nerve conduction velocities are calculated for each segment by dividing the distance between two adjacent stimulus and recording sites, or two stimulus sites, by the latency (conduction time) between these same sites. Sensory nerve responses are considerably smaller than motor responses. Their amplitudes are generally measured in microvolts (μV). Sensory recordings are more sensitive than motor recordings in cases of mixed sensory-motor neuropathies. When a motor nerve is stimulated in the periphery, both orthodromic (peripherally to the muscle) and antidromic (centrally toward the spinal cord) impulses are generated. A proportion of the antidromic impulses will, as it were, “bounce off” the axon hillock and return as a recurrent discharge along the same neurons to activate the muscle from which the recording is taken. This activity is termed the F wave (Figure 33-4), and it is observed as a small wave occurring after the M wave.11,13,14 No synapse is involved. Thus the F wave is not a reflex response, but rather only a measure of conduction along the motor neuron. Specific conditions of electropotential must exist at the soma-dendritic cell membrane to reactivate the efferent axon; therefore the occurrence of the F-wave response is inconsistent and variable in latency and waveform.15 The F-wave latency can be useful in evaluating conduction in conditions usually involving the proximal portions of the peripheral neurons (e.g., radiculopathy, Guillain-Barré syndrome [refer to Chapter 17], or thoracic outlet syndrome [refer to Chapter 18]). Its value, however, has been questioned by some authors because of its variability. Normal values of F-wave latency are 22 to 34 ms in the upper extremity (stimulating at the wrist) and 40 to 58 ms in the lower extremity (stimulating at the ankle), depending on the height of the subject, with a bilateral difference in latency of no greater than 1 ms.16 The H-reflex response latency (Figure 33-5) is a measure of the time for action potentials elicited by stimulating a nerve in the periphery to be propagated centrally over the Ia afferent neurons to the spinal cord, to be transmitted across the synapse to alpha motor neurons, and then to travel distally over these neurons to activate the muscle. The response therefore measures conduction in both the afferent and efferent neurons.11,13 It is also referred to as a “late” response (the other being the F wave). The H reflex is constant in latency and waveform, and it occurs with a stimulus usually below the threshold level required to elicit the M-wave response (Ia afferent fibers are larger in diameter than alpha motor neurons and thus more sensitive to electrical stimulation). This monosynaptic reflex response is most easily found by stimulating the tibial nerve at the popliteal area and recording from the soleus muscle. Braddom and Johnson17 reported a mean latency of 29.8 ms (±2.74 ms) for the tibial nerve in normal adults, and a bilateral difference of no more than 1.2 ms. The H-reflex latency is a valuable measure of conduction over the S1 nerve root in differentiating suspected proximal plexopathy and radiculopathy from a herniated disc or foraminal impingement. Sabbahi and Khalil18 have reported a technique for recording the H reflex from the flexor carpi radialis muscle when stimulating the median nerve. In normal human beings older than 1 year, the H reflex is usually seen only in the tibial, femoral, and median nerves. It can be elicited from several nerves in infants and in conditions of CNS dysfunction in adults. The repetitive nerve stimulation (RNS) test is used to evaluate transmission at the neuromuscular junction (motor synapse) in patients with diffuse weakness. RNS tests are helpful in the differential diagnosis of disorders such as myasthenia gravis and Lambert-Eaton myasthenic syndrome (LEMS). One protocol uses a series of supramaximal electrical stimuli applied to a peripheral nerve at a distal site (e.g., median or ulnar nerve at the wrist) at a rate of three to five per second for five to seven responses. Changes in amplitude of the muscle response are assessed. Precise technical requirements are specified to prevent movement artifacts and other testing errors. Detailed descriptions of the RNS test can be found in other texts.11,19 Under normal conditions the amplitude does not change more than 10% from that of the initial response in a series of 10 stimuli recorded before and after resistive exercise. An amplitude decrease in the fifth or sixth response of more than 10% is considered abnormal and is compatible with a physiological defect at the postsynaptic receptor site of the neuromuscular junction, as in myasthenia gravis. In another RNS protocol, stimuli are applied to a nerve, first at a slow rate, then at a faster rate, usually 10 to 20 per second for up to 10 seconds. Normally, the amplitude can decrease up to 40% from the initial amplitude. In some defects at the presynaptic site, the response may be lower than normal during a slow stimulation rate but show a significant amplitude increase at the higher rate. Increases in amplitude greater than 100% over the initial response are consistent with presynaptic neuromuscular junction defects such as seen in LEMS, which has a strong association with small-cell bronchogenic carcinoma, and in botulism. In 1957 Eaton and Lambert20 reported this phenomenon as a myasthenic syndrome. Gilchrist and Sanders21 reported another protocol referred to as a double-step RNS test. This test measures amplitude before and after a temporarily induced ischemia of the extremity. They found the double-step RNS test to be slightly more sensitive than the routine RNS test, but only 60% as sensitive as the SFEMG technique. The RNS test is a good alternative test for neuromuscular transmission when the SFEMG is not available, but the examiner must meticulously adhere to technical details when conducting the test. Hence, the blink reflex is a nerve conduction test that assesses a portion of the CNS and has shown some utility in assisting in the diagnosis of multiple sclerosis (see Chapter 19) and Wallenberg syndrome.7 Electrical potentials elicited by stimulation of nerves or sense organs in the periphery can be recorded from various sites as the impulses are transmitted centrally along the neuronal pathway and from the representative area of the brain.11,22–24 SSEP procedures are particularly useful in assessing the integrity of afferent pathways in the CNS. They are helpful in differentiating among lesions in areas such as the plexus, spinal cord, brain stem, thalamus, and cerebral cortex. Evoked potential tests have the advantage of providing data about the integrity of both peripheral and central neuronal pathways, including transmission across axodendritic synapses. In visual evoked potential (VEP) procedures, visual stimuli such as variable light flashes of changing patterns are applied to one or both eyes under highly controlled conditions. The response is recorded from the scalp over the representative area of the cerebral cortex.11,23,24 The term pattern reversal evoked potentials (PREPs), a more descriptive term for these procedures, is recommended by the American Electroencephalographic Society.22 These tests and other VEP procedures are useful in assessing pathology of retinal photoreceptors, the optic nerve, and postchiasmal pathways. Abnormal conduction findings have been reported when VEP studies are used in demyelinating disorders such as multiple sclerosis and optic neuritis. The examiner may conclude that the patient is cortically blind because no response is recorded on the visual cortex. Although many causes for a stimulus not reaching the visual cortex are possible, the end result is considered blindness. If the cause for cortical inactivity is swelling or a neurochemical imbalance within a nuclear relay structure, once corrected, the individual may experience normal vision. A change in the reaction of the patient to the visual environment may reflect increased awareness and a change in coma scale rating. Similarly, just because an individual turns toward a light or visual stimulus does not mean an evoked potential reaches the visual cortex. Instead, the eyes as receptors and the visual tract to the brain stem may be intact even though a problem in the synaptic connections between or within the thalamus and visual cortex may exist. Auditory evoked potential tests are used to evaluate neurological function of the cochlear division of the auditory nerve (eighth cranial nerve), central auditory pathways and synapses in the brain stem, and the receptor areas on the cerebral cortex.11,22–24 Brain stem auditory evoked potentials are frequently referred to as BAEPs. A series of high-intensity clicks is applied to auditory receptors in the ears through headphones, and several components of the response waveforms are recorded by using surface electrodes over the representative cortical areas. The BAEP is an effective test procedure for localizing and evaluating acoustic neuromas and other space-occupying lesions in the brain stem. This test is also used for assessment of brain damage in patients who are comatose as a result of traumatic brain injury (TBI). Robinson and Rudge25 recommend caution in using BAEP tests for this purpose because other factors, such as defective receptor organs, can cause abnormalities in BAEPs. The evoked potential tests described in this chapter all require application of appropriate external stimuli that are rapidly repeated many times. The response is electronically averaged to sort out the desired signal from interference signals. The conduction times (latencies), waveform shape and amplitude, and sometimes conduction velocities are measured and compared with normal values. Absence of a response, increased latencies, decreased amplitudes, and slowing of conduction velocities are all abnormal findings. Normal values and details of techniques for the evoked potential tests are described elsewhere.11,22–24 Unlike NCSs, which use the electrical stimulation of the motor nerves to elicit muscle contraction, the needle EMG is used to record and analyze muscle activity at rest and during voluntary activation. It is particularly useful in identifying pathology of the lower motor neurons and of the muscle itself. EMG can also be used to identify abnormalities of motor neuron recruitment that are associated with certain disorders of the CNS especially when NCS findings are normal—as would be the case in radiculopathies. The primary recording studied is the insertional activity, along with activity at rest and the motor unit action potential (MUAP), which is produced by the depolarization of single motor units during voluntary or reflex activity. Spontaneous electrical activity of single muscle fibers at rest is termed fibrillation and is diagnostic of denervation. For recording of muscle activity, small-diameter needles are inserted within the muscles to be studied. Three electrodes are required: active (negative), reference (positive), and ground. The needles may be monopolar, requiring a second needle or surface electrode for reference, or bipolar, containing both the active and reference electrodes (usually concentric in cross section). The ground electrode is typically placed on the surface of the skin. Most commonly the needles used are disposable. The activity detected in the muscle is displayed on the video display terminal of a computer (and can be stored and printed later). It is simultaneously played through an audio amplifier. The electromyographer can often identify pathological conditions by the characteristic “sounds” of the electrical activity of the muscle. Many excellent resources are available for readers interested in details of the equipment and procedures for EMG.9–11,26,27 Details of contraindications and special precautions are described by Currier and colleagues.27 Needle EMG is sensitive in determining the state of the axons. When axons are interrupted, there is denervation of the muscle cell. The findings in denervation and partial denervation include increased insertional activity, fibrillation potentials or positive sharp waves at rest, and a reduced or absent interference pattern. CNS dysfunction can result in no resting potentials, but if motor control was impaired a decreased or abnormal interference pattern might be apparent because of difficulty in recruitment (Figure 33-6). Needle EMG also informs the clinician about muscle cell disorders such as myopathies. The primary finding in the case of a myopathy is fibrillation potentials and small-amplitude polyphasic MUAPs. • Amplitude: Low amplitude is seen in myopathy or nascent potential; large amplitude is a sign of chronicity. • Phases: Normal MUAPs are biphasic or triphasic. Polyphasia is indicative of denervation-reinnervation. • Recruitment pattern: A less-than-full recruitment is indicative of fewer motor units discharging, as can be seen in axon loss. • Firing rate: If elevated, fewer motor units are contracting more often to provide the same tension in a denervated muscle. Instruments with computer-assisted analysis are now commonplace for studying electromyographic signals in great detail.11,28–30 Parameters of the waveform, including amplitude, duration, frequency spectrum, number of turns, or phase polarity reversals and area (the integral or total voltage of the waveform) can be automatically analyzed. The data are then compared electronically with predetermined patterns of electrical changes, which correlate with categories of neuromuscular disorders such as myelopathies and neuropathies. The following is a summary of the more characteristic EMG and nerve conduction changes associated with selected groupings of neurological disorders. The intent is to assist in the understanding of reports of these studies and recognize changes that may be seen in sequential tests during the course of the disorders. The following is a simplified grouping of electrical changes; actual electrodiagnostic studies show considerably more detail and frequent variations of these findings.9–11,26,27 Electrical testing in CNS disorders typically shows normal motor and sensory nerve conduction. In the EMG, spontaneous activity is typically not seen, and individual motor units seen on muscle contraction usually have normal parameters. The recruitment pattern may show a slower-than-normal MUAP discharge frequency with an incomplete and irregular interference pattern. In the presence of tremor and other involuntary movements, bursts of MUAPs occur, consistent with the muscle contraction pattern. In cases involving the brain stem, the blink reflex may show abnormalities.7 The tests are important in differential diagnosis between a CNS and a PNS problem, but often they are not used when clinical examinations demonstrate the problem to be definitively in the CNS. In myelopathies, which include upper and lower motor neuron disorders (e.g., amyotrophic lateral sclerosis [ALS], poliomyelitis, cervical spondylitis, and syringomyelia), motor and sensory nerve conduction is usually normal, although mild slowing may be present.11 The characteristic EMG changes, which usually appear in the more chronic stages of the disorders, are increased amplitude and duration of MUAPs because of the variable impulse conduction time in sprouting axon terminals. An increased number of polyphasic potentials with increased duration is usually found. Spontaneous activity is often seen, and on strong contraction fewer rapidly firing large MUAPs are recruited, resulting in a single-unit or partial interference pattern. In ALS, fasciculations and denervation potentials are typically found. The distribution of the EMG abnormalities determines the extent of the condition. Peripheral neuropathies show a variety of electrical changes depending on the type and location of the pathology. In a proximal pathology (e.g., radiculopathy), motor and sensory nerve conduction generally remain normal, except F waves and H-reflex responses in specific spinal segments. If motor nerve roots are compromised, spontaneous activity and increased polyphasic potentials appear, and reduced recruitment of MUAPs results in an incomplete interference pattern. In more chronic stages MUAP amplitude and duration can be increased. As the lesion improves, spontaneous activity decreases and the recruitment patterns become more normal. If only sensory roots are injured, no EMG changes occur. Again, the distribution of the EMG abnormalities (all in one myotome) is pathognomonic, especially in the presence of denervation potentials in the corresponding paraspinal muscles.31 Generalized, systemic peripheral polyradiculoneuropathies can be divided into primarily demyelinating, primarily axon loss, or mixed axonal-demyelinating polyneuropathies. Some involve mostly sensory nerves (e.g., hereditary sensory neuropathy types I to IV, Sjögren syndrome, Friedreich ataxia), and others mostly motor nerves (e.g., chronic inflammatory demyelinating polyneuropathy [CIDP], lead neuropathy), but most involve both sensory and motor nerves. In the primarily demyelinating type, such as Guillain-Barré syndrome, motor and sensory nerve conduction and F waves become markedly slow. EMG changes usually do not occur, except for a reduced recruitment pattern consistent with weak muscle contraction or conduction block (when the demyelination is such that the impulse does not propagate). With primarily axonal polyneuropathies, such as uremic neuropathy, isoniazid or cisplatin toxicity, and lead poisoning, motor and sensory nerve conduction is mildly slowed or may remain normal. The duration and amplitude of the response, however, decrease. During advanced stages, many polyneuropathies develop both demyelinating and axonal pathology (e.g., diabetic neuropathy, which is by far the most commonly encountered polyneuropathy). On EMG, spontaneous activity is commonly seen. These electrical changes generally become more severe with worsening of the pathology, but they also improve if the pathology is reversed. From a patient management standpoint, remyelination occurs at a much more expedient pace than reinnervation.32–34 Table 33-1 provides a summary of typical findings. TABLE 33-1
Electrophysiological testing and electrical stimulation in neurological rehabilitation
Electrophysiological testing
Anatomical review
At the cellular level
At the anatomical level
Nerve conduction tests
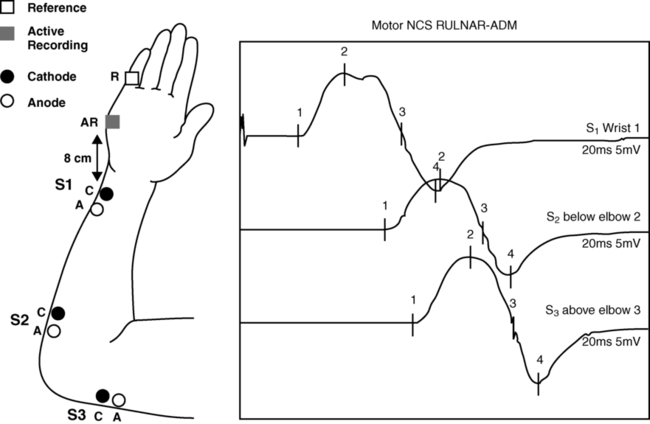
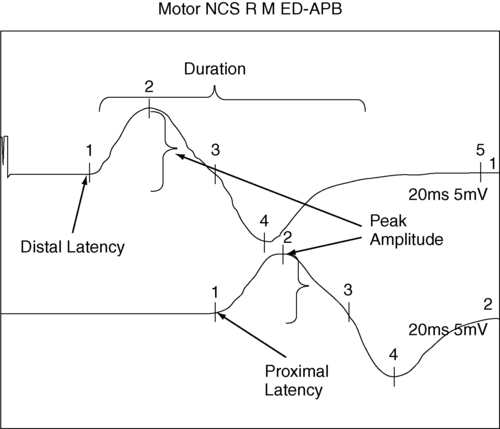
Motor nerve conduction
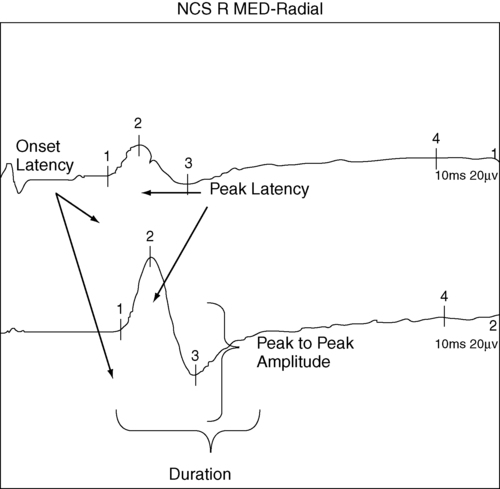
Sensory nerve conduction
 Example of recording and stimulation sites.
Example of recording and stimulation sites.
F-wave latency
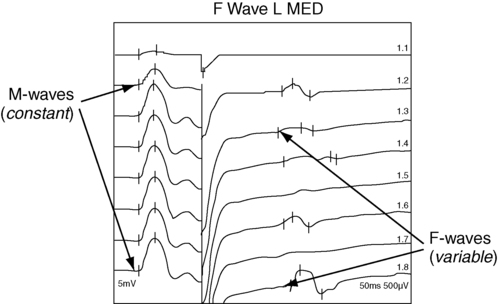
 Example of F-wave study.
Example of F-wave study.
H-reflex response
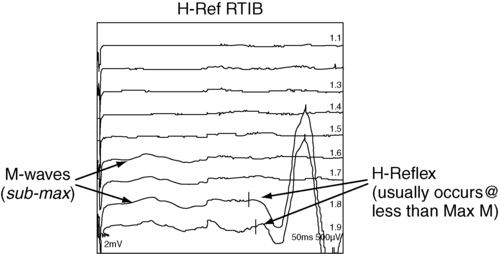
 Example of H-reflex study.
Example of H-reflex study.
Repetitive stimulation tests
Blink reflex
 Trigeminal neuralgia (cranial nerve V) or Guillain-Barré: all responses delayed
Trigeminal neuralgia (cranial nerve V) or Guillain-Barré: all responses delayed
 Acoustic neuroma or Bell palsy (cranial nerve VII): ipsilateral responses delayed
Acoustic neuroma or Bell palsy (cranial nerve VII): ipsilateral responses delayed
 Medullar lesion: absence of R2
Medullar lesion: absence of R2
Clinical evoked potentials
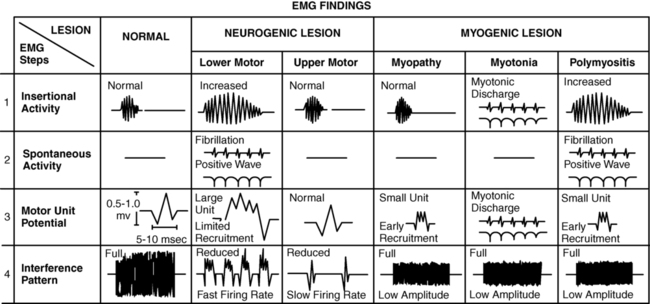
Needle electromyography
 Insertional activity: may be increased in acute denervation and myopathic processes
Insertional activity: may be increased in acute denervation and myopathic processes
 Spontaneous activity at rest: present (positive sharp waves and fibrillation potentials) in denervation
Spontaneous activity at rest: present (positive sharp waves and fibrillation potentials) in denervation
 Fasciculations: may be present in motor neuron disorders
Fasciculations: may be present in motor neuron disorders
Summary of clinical electroneuromyographic and nerve conduction studies

DISORDER
MOTOR CONDUCTION
SENSORY CONDUCTION
ELECTROMYOGRAPHY
Motor neuron disease (e.g., amyotrophic lateral sclerosis [ALS])
Reduced amplitudes
Normal
Acute plus chronic neurogenic changes, fasciculations
Radiculopathies
Normal
Normal
Acute neurogenic changes in myotome
Plexopathies
Reduced amplitudes
Reduced amplitudes
Acute neurogenic changes in specific pattern
Axonal neuropathy
Reduced amplitude in affected nerve(s)
Reduced amplitude in affected nerve(s)
Acute neurogenic changes in affected nerve
Demyelinating neuropathy
Reduced conduction in affected nerve
Reduced conduction in affected nerve
Normal
Neuromuscular junction disorder
Decrement (myasthenia gravis [MG]) or increment (Lambert-Eaton myasthenic syndrome [LEMS]) with repetitive stimulation
Normal
Occasional myopathic motor unit action potentials (MUAPs)
Myopathies
Normal
Normal
Small-amplitude polyphasic MUAPs ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Electrophysiological testing and electrical stimulation in neurological rehabilitation
Only gold members can continue reading. Log In or Register to continue