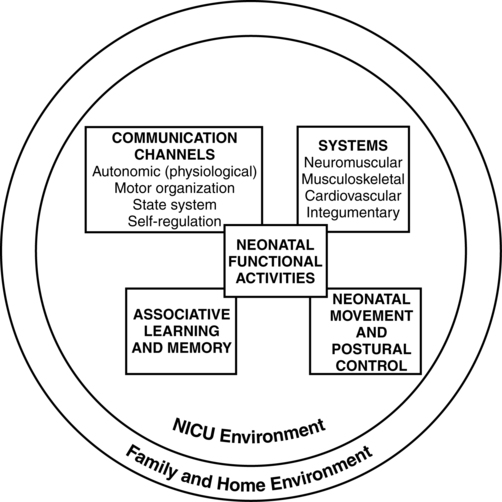
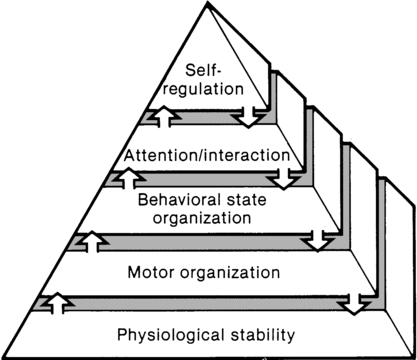
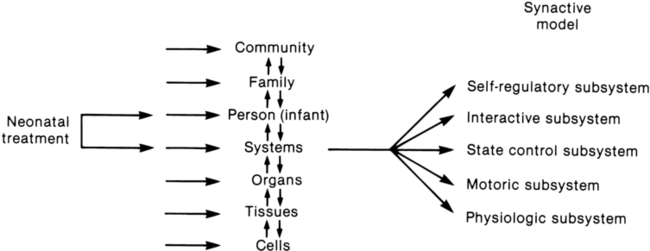
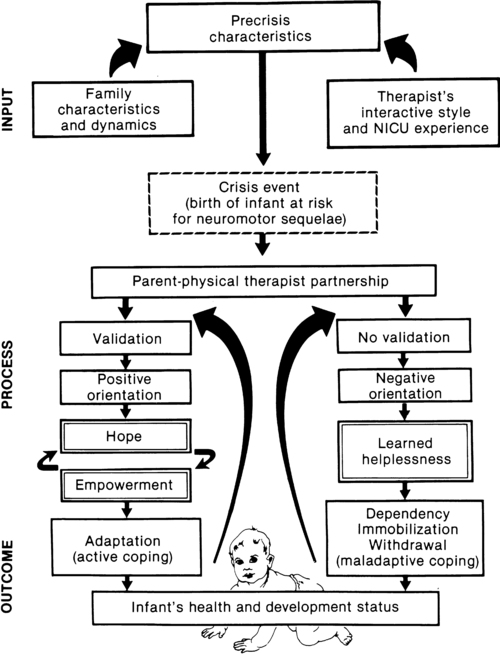
JANE K. SWEENEY, PT, PhD, PCS, C/NDT, FAPTA, TERESA GUTIERREZ, PT, MS, PCS, C/NDT and JOANNA C. BEACHY, MD, PhD, FAAP After reading this chapter the student or therapist will be able to: 1. Discuss three theoretical frameworks guiding neonatal therapy services in the neonatal intensive care unit. 2. Identify the physiological and structural vulnerabilities of preterm infants that predispose them to stress during neonatal therapy procedures. 3. Outline supervised clinical practicum components and pediatric clinical experiences to prepare for entry into neonatal intensive care unit practice. 4. Describe how the grief process may affect behavior and caregiving performance of parents of low–birth-weight neonates. 5. Differentiate the developmental course and neuromotor risk signs in infants with emerging neuromotor impairment from the clinical characteristics of infants with transient movement dysfunction. 6. Identify instruments for neuromotor examination of high-risk infants in neonatal intensive care units and in follow-up clinics and compare psychometric features of the tests. 7. Describe program plans and follow-up for low–birth-weight infants in neonatal intensive care unit and home settings. Premature birth is associated with an increased prevalence of major and minor neurodevelopmental disability. Advancements in newborn resuscitation and neonatal intensive care have contributed to greatly improved survival of infants with low birth weight (LBW), but risk of neurodevelopmental sequelae remains high.1,2 Although brain injury can be documented by ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) in infants, prediction of subsequent neurodevelopmental outcome is still relatively unreliable.3–6 Serial clinical examinations and careful monitoring of neurodevelopmental status are therefore critical during the neonatal period and discharge from the neonatal intensive care unit (NICU) through the outpatient phase of care. Pediatric therapists with mentored, subspecialty training in neonatology and infant therapy approaches can serve these increasing numbers of surviving neonates at neurodevelopmental risk by (1) providing valuable diagnostic data through neurological and developmental examination, (2) participating in developmental and environmental interventions adapted to each infant’s physiological, motor, and behavioral needs, (3) facilitating and coordinating interdisciplinary case management for infants and parents, and (4) reinforcing preventive aspects of health care through early intervention and long-term developmental monitoring. Dynamic systems theory applied to infants in the NICU refers first to the presence of multiple interacting structural and physiological systems within the infant to produce functional behaviors and second to the dynamic interactions between the infant and the environment. In Figure 11-1, neonatal movement and postural control are targeted as a core focus in neonatal therapy, with overlapping and interacting influences from the cardiopulmonary,7 behavioral, neuromuscular, musculoskeletal, and integumentary systems. A change or intervention affecting one system may diminish or enhance stability in the other dynamic systems within the infant. Similarly, a change in the infant’s environment may impair or improve the infant’s functional performance. This theory guides the neonatal practitioner to consider the many potential physiological and anatomical influences (dynamic systems within the infant) that make preterm infants vulnerable to stress during caregiving procedures, including neonatal therapy. In dynamic systems theory, emphasis is placed on the contributions of the interacting environments of the NICU, home, and community in constraining or facilitating the functional performance of the infant.8 The synactive model of infant behavioral organization is a specific neonatal dynamic systems model for establishing physiological stability as the foundation for organization of motor, behavioral state, and attention or interactive behaviors in infants. Als and colleagues9–11 described a “synactive” process of four subsystems interacting as the neonate responds to the stresses of the extrauterine environment. They theorized that the basic subsystem of physiological organization must first be stabilized for the other subsystems to emerge and allow the infant to maintain behavioral state control and then interact positively with the environment (Figure 11-2). To evaluate infant behavior within the subsystems of function addressed in the synactive model, Als and colleagues10,11 developed the Assessment of Preterm Infants’ Behavior (APIB). With the development of this assessment instrument, a fifth subsystem of behavioral organization, self-regulation, was added to the synactive model. The self-regulation subsystem consists of physiological, motor, and behavioral state strategies used by the neonate to maintain balance within and between the subsystems. For example, many infants born preterm appear to regulate overstimulating environmental conditions with a behavioral state strategy of withdrawing into a drowsy or light sleep state, thereby shutting out sensory input. The withdrawal strategy is used more frequently than crying because it requires less energy and causes less physiological drain on immature, inefficient organ systems. Fetters12 placed the synactive model within a dynamic systems framework to demonstrate the effect of a therapeutic intervention on an infant’s multiple subsystems (Figure 11-3). She explained that although a neonatal therapy intervention is offered to the infant at the level of the person, outcome is measured at the systems level, where many subsystems may be affected. For example, the motor outcome from neonatal therapy procedures is frequently influenced by “synaction,” or simultaneous effects, of an infant’s physiological stability and behavioral state. Physiological state and behavioral state are therefore probable confounding variables during research on motor behavior in neonatal subjects. Neonatal therapists may find this combined dynamic systems and synactive framework helpful in conceptualizing and assessing changes in infants’ multiple subsystems during and after therapy procedures. a major component of the intervention process in neonatal therapy is the interpersonal helping relationship with the family. A hope-empowerment framework (Figure 11-4) may guide neonatal practitioners in building the therapeutic partnership with parents; facilitating adaptive coping; and empowering them to participate in caregiving, problem solving, and advocacy. The birth of an infant at risk for a disability, or the diagnosis of such a disability, may create both developmental and situational crises for the parents and the family system. The developmental crisis involves adapting to changing roles in the transition to parenthood and in expanding the family system. Although not occurring unexpectedly, this developmental transition for the parents brings lifestyle changes that may be stressful and cause conflict.13 Because parents are experiencing (mourning) loss of the “wished for” baby they have been visualizing in the past 6 months, they often struggle with developing a bond with their “real” baby in the NICU.14 A situational crisis occurs from unexpected external events presenting a sudden, overwhelming threat or loss for which previous coping strategies either are not applicable or are immobilized.15 The unfamiliar, high-technology, often chaotic NICU environment creates many situational stresses that challenge parenting efforts and destabilize the family system.16 The language of the nursery is unfamiliar and intimidating. The sight of fragile, sick infants surrounded by medical equipment and the sound of monitor alarms are frightening. The high frequency of seemingly uncomfortable, but required, medical procedures for the infant are of financial and humanistic concern to parents. No previous experiences in everyday life have prepared parents for this unnatural, emergency-oriented environment. This emotional trauma of unexpected financial and ongoing psychological stresses during parenting and caregiving efforts in the NICU contributes to potential posttraumatic stress disorder in parents of infants requiring intensive care.17,18 The quality and orientation of the helping relationship in neonatal therapy affect the coping style of parents as they try to adapt to developmental and situational crises (see Figure 11-4). Although parents and neonatal therapists enter the partnership with established interactive styles and varying life and professional experiences, the initial contacts during assessment and program planning set the stage for either a positive or a negative orientation to the relationship. Despite many uncertainties about the clinical course, prognosis, and quality of social support, a positive orientation is activated by validation or acknowledgment of parents’ feelings and experiences. Validation then becomes a catalyst to a hope-empowerment process in which many crisis events, negative feelings, and insecurities are acknowledged in a positive, supportive, nonjudgmental context in which decision-making power is shared.19 In contrast, a negative orientation may be inadvertently facilitated by information overloading without exploration and validation of parents’ feelings, experiences, and learning styles. This may lead to magnified uncertainty, fear, and powerlessness with the misperception of excessive complexity in the proposed neuromotor intervention activities. In a hope-empowerment framework, parent participation in neuromotor intervention allows sharing of power and responsibility and promotes continuous, mutual setting and revision of goals with reality grounding. Adaptive power can be generated by helping parents stabilize and focus energy and plans and by encouraging active participation in intervention and advocacy activities.19 Exploring external power sources (e.g., Parent to Parent USA or other parent-to-parent support groups20) early in the therapeutic relationship may help parents focus and mobilize.20–23 Hope and empowerment are interactive processes. They are influenced by existential philosophy: the hope to adapt to what is and the hope to later find peace of mind and meaning for the situation, regardless of the infant’s outcome. In describing the effect of a prematurely born infant on the parenting process, Mercer24 related that “hope seems to be a motivational, emotional component that gives parents energy to cope, to continue to work, and to strive for the best outcome for a child.” She viewed the destruction of hope as contributing to the physical and emotional withdrawal frequently observed in parents who attempt to protect themselves from additional pain and disappointment and then have difficulty reattaching to the infant. Hope contributes to the resilience parents need to get through the arduous 1- to 4-month NICU hospitalization period and then begin to face the future in their home and community with an infant at neurodevelopmental risk. Groopman25 proposed that hope provides the courage to confront obstacles and the capacity to surmount them. He described the process of creating a middle ground where truth (of the circumstances) and hope reside together as one of the most important and complex aspects in the art of caregiving. Improvements in neonatal intensive care over the last 30 years have led to the increased survival of preterm and term infants. Specific obstetric advances include establishment of specialized tertiary care centers, earlier identification of high-risk pregnancies, improvements in prenatal diagnosis, and medications used to stabilize maternal medical conditions and enhance fetal well-being. Respiratory compromise in preterm infants has significantly decreased as a result of (1) maternal betamethasone administration to promote fetal lung maturity; (2) availability of commercial surfactant to improve pulmonary function; and (3) advances in ventilator design and capability, enhancing management of respiratory distress with significantly diminished pulmonary dysfunction. In addition, improvements in continuous monitoring of vital signs, radiological imaging techniques, delivery of medications, and maintenance of thermal stability have aided earlier identification of neonatal problems and enhanced improvements in care. Increased survival is most evident in the extremely low–birth-weight (ELBW) infant, that is, birth weight less than 1000 g. For infants born at 23 weeks of gestation, survival has increased from approximately 0% to more than 50%, and for infants born at 26 weeks of gestation, survival has increased from 25% to 85%.26 It is important to note that the incidence of severe neurological injury has decreased over time in these extremely preterm infants. However, a significant number of preterm infants will exhibit long-term neurological impairment owing to increased survival. The long-term effect of a neurological insult on the developing brain depends on the timing of the injury, the gestational age of the infant, and the nature and duration of the insult.27 During the first month of gestation, the neural tube is formed. Neurological insult at this time leads to abnormal neural tube development, specifically anencephaly, encephalocele, or myelomeningocele. Neuronal proliferation is nearly complete by 5 months of gestation. All neurons and glial cells originate in the ventricular and subventricular zone (germinal matrix). Disorders of proliferation result in microcephaly, with either decreased size or decreased number of proliferating neuronal units or macrocephaly. Neuronal migration occurs at 3 to 6 months of gestation, and neurons are guided by glial cells to form neuronal columns. Subplate neurons, essential for correct organization of the brain, are formed at this time. Insults during this period of development result in marked disturbance of neurological structure and function with aberrations noted in gyral formation (lissencephaly, schizencephaly, and polymicrogyria) and/or absence of the corpus callosum. The final phase of brain organization is glial maturation to astrocytes and oligodendrocytes. Astrocytes help maintain the blood-brain barrier, provide nutrient support, regulate neurotransmitter and potassium concentration, and assist in neuronal repair after injury. Oligodendrocytes produce myelin, a protective fatty sheath that surrounds axons (white matter) and facilitates nerve transmission. Myelination starts in midgestation and continues through adulthood. Oligodendrocytes are especially sensitive to hypoxia and other insults. Disruption of normal myelination results in white matter hypoplasia and periventricular leukomalacia (PVL) (see later discussion) leading to impaired motor function.27 The most common neonatal problems associated with impaired neurological functioning and long-term developmental delay are listed in Table 11-1. In addition to descriptions of neonatal neurological conditions, a discussion is provided on the impact on neonatal development of maternal medication, such as drugs of abuse and psychotropic medications. The importance of developmental follow-up for healthy “late” preterm infants born at 34 to 366/7 weeks of gestation is also discussed in this section. TABLE 11-1 NEONATAL COMPLICATIONS AFFECTING BRAIN DEVELOPMENT IVH originates in the microcirculation or capillary network of the germinal matrix.28 The germinal matrix is located adjacent to the ventricle and is a well vascularized area owing to the high metabolic demand from the rapidly proliferating neuronal stem cells. Vessels in the germinal matrix are thin walled and fragile, which predisposes them to rupture. In addition, preterm infants have impaired autoregulation—that is, the inability to maintain cerebral blood flow across a large range of blood pressures. Thus during labor, delivery, and the immediate postpartum transition period, changes in blood pressure can lead to cerebral hypoperfusion and ischemia as well as to hyperperfusion and vessel rupture. Alterations in CO2 lead to either reduced cerebral blood flow from hypocarbia or increased flow from hypercarbia. Other risk factors for IVH include asphyxia, fluid bolus infusion (especially of hypertonic solutions), anemia, and pain.29 Platelet and coagulation disturbances have been implicated as risk factors for the development of IVH. IVH is rarely seen in infants with gestational age greater than 32 weeks owing to the developmental involution of vessels in the germinal matrix. Diagnosed by cranial ultrasound, IVH is graded in severity from 1 to 4,28 with grade 1 IVH being the most mild because the hemorrhage is confined to the germinal matrix. In grade 2 IVH, the hemorrhage extends into the ventricle (Figure 11-5). Grade 3 IVH occurs when the hemorrhage fills more than 50% of the ventricle and causes ventricular distention. Grade 4 IVH, or periventricular hemorrhagic infarct (PVHI), is a complication of IVH caused by venous congestion of the terminal veins that border the lateral ventricles leading to white matter necrosis (see later).30 It is important to note that IVH may not be apparent on cranial ultrasound in the first few days after birth. However, 90% of IVHs can be detected by day 4. In addition, the full extent of the hemorrhage may not be appreciated for several days after the initial diagnosis of IVH is made.28 The evolution of grades 3 and 4 IVH over 10 days is shown in Figure 11-6, A and B. Many researchers have investigated the relationship of IVH grades with severity of neurodevelopment delay. In general, grades 1 and 2 IVH are not associated with a significant increase in developmental abnormalities but do not ensure normalcy. Infants with severe IVH (grade 3 and/or 4) have increased mortality and are at markedly increased risk for developmental disabilities, specifically spastic hemiplegia or diplegia affecting the lower extremities. As can be seen in Figure 11-7, motor tracts innervating the lower extremities are in close proximity to the area of the germinal matrix and the site of the origin of IVH leading to lower-extremity spastic cerebral palsy (CP). However, abnormalities visible on cranial ultrasound are not able to absolutely predict long-term outcome because the amount of cortex damaged and the neuronal tracts affected by IVH cannot be identified by ultrasound. In addition, ultrasound may not be sensitive enough to identify PVL (see later). Grade 4 IVH was originally thought to be an extension of IVH into the parenchyma but is actually a known complication of IVH.30 PVHI is caused by venous compression of the terminal veins that border the lateral ventricles leading to impaired venous drainage and congestion and eventually hemorrhagic infarction. The usual initial distribution of PVHI seen on cranial ultrasound is fan-shaped echodensities in the periventricular location (see Figure 11-6, A2-4). Over time there is destruction of preoligodendrocytes and motor axons leading to white matter necrosis and the development of porencephalic cyst. PVHI is usually unilateral (approximately 70%), and approximately three quarters of cases are associated with severe IVH [grade 3 and 4]. Infants with small, unilateral PVHI have no increased risk of developmental delays compared with infants with grade 3 IVH. However, if the PVHI is bilateral or if multiple porencephalic cysts are present, the risk of severe motor impairment and CP is significantly increased. In addition, approximately 50% of infants with PVHI have visual field defects, probably secondary to damage to the axons of nerves carrying information to the visual cortex.30 Approximately 50% of infants with severe IVH will develop posthemorrhagic ventricular dilatation (PHVD) from either blockage of the normal flow of cerebrospinal fluid (CSF) or decreased absorption of CSF. Approximately 50% to 75% of these infants will develop progressive PHVD, resulting in need for treatment. The severity of ventricular dilatation can be measured via serial cranial ultrasound examinations.31,32 Severe ventricular dilatation is usually evident by 2 to 3 weeks after birth. Rapid increase in head circumference does not occur until approximately 4 weeks after birth.33 Posthemorrhagic ventricular dilatation is treated by serial removal of CSF by spinal tap, subgaleal shunt, or placement of an Ommaya reservoir. Removal of CSF has been shown to decrease intracranial pressure and improve cerebral perfusion34 and also to increase cortical gray and white matter.35 In addition, there is indirect evidence that ventricular distention itself may cause secondary brain injury through stretching and disruption of axons, gliosis, and loss of oligodendrocytes. No consensus has been reached on the optimal management of PHVD. Ventriculoperitoneal (VP) shunt placement is necessary in infants with PHVD that does not resolve with serial removal of CSF. Significant complications of VP shunt include sepsis, specifically ventriculitis, or shunt malfunction such as blockage or failure. These complications necessitate shunt revisions, which further compromise these fragile infants. Researchers from a consortium of 17 tertiary NICUs recently published results on the neurodevelopmental outcome at 2 years of age of ELBW infants with grade 3 and 4 IVH who were born from 1993 to 2002.36 Infants who required VP shunt placement had significantly worse outcomes than infants with grade 3 or 4 IVH alone (Table 11-2). Moreover, the number of infants who were untestable (MDI or PDI = 49) because of severe neurodevelopmental handicap was significantly increased in the group of infants who received a VP shunt. In addition, CP was significantly more prevalent in infants who had a VP shunt placed than in infants with only grade 3 or 4 IVH. TABLE 11-2 CP, Cerebral palsy; MDI, Mental Developmental Index; PDI, Psychomotor Development Index. Modified from Adams-Chapman I, Hansen NI, Stoll BJ, et al: Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121:1167-1177, 2008. Recent retrospective studies from the Netherlands indicated that earlier intervention when the ventricles are moderately dilated significantly decreased the need for VP shunt from 62% to 16% and trended to improve long-term developmental outcome with a decreased incidence of moderate to severe handicap.32,37 Thus, halting the progression of PHVD and decreasing the need for VP shunt is likely to improve long-term outcome in these infants. However, because PVHD can spontaneously resolve without intervention, identification of factors that can accurately predict which infant will develop persistent PHVD and consequently require VP shunt placement is needed. PVL can be either cystic (see Figure 11-6, C) or global and may be difficult to identify on radiological images. Cystic PVL results from the focal dissolution of cellular tissue approximately 3 weeks after the insult and can be identified on ultrasound if greater than 0.5 cm in diameter.27 However, cysts visualized by cranial ultrasound may disappear over time owing to fibrosis and gliosis. Thus the incidence of cystic PVL is felt to be underestimated by cranial ultrasound examination. Global PVL results from diffuse white matter injury and myelin loss. This finding can be subtle with moderate ventricular dilatation and/or a mild increase in extraaxial fluid on cranial imaging. Infants with severe PVL have marked ventricular dilatation, increased extraaxial fluid, and decreased head growth. MRI obtained at term is more sensitive in identifying white matter injury than cranial ultrasound and is predictive of subsequent neurosensory impairment and cognitive delay present in up to 50% of extremely preterm infants.38 Newer techniques, such as diffusion tensor imaging (DTI), functional connectivity MRI (fcMRI), and morphometry for analysis of cortical folding are being investigated as early markers of impaired neurodevelopmental outcome. Diffusion tension imaging measures the restriction of water diffusion in the myelin sheath surrounding axons and yields information at the microstructure level about axon caliber changes and aberrations in myelination. In addition, DTI allows for visualization of brain fiber tracks and neuronal connectivity. In research, fMRI is used to investigate interaction between areas of the brain at rest and during tasks by analyzing changes in blood flow. Morphometic analysis of sequential MRI scans has been used to create maps of cortical folding with quantification of surface area and degree of gyral formation. White matter injury results in delayed myelination and altered cortical folding.38 Both fMRI and morphometric analysis of cortical folding are currently available only in research studies, not in clinical management. Complications of NEC include sepsis, wound infection, and stricture formation (10% to 35%) requiring repeated surgery. Growth of infants with NEC can be impaired due to feeding intolerance, prolonged total parenteral nutrition (TPN), removal of significant amounts of intestine, and repeated surgeries and infections. Persistence of weight at less than 10% for age is correlated with poor neuromotor and neurodevelopmental outcome.39 Failure to achieve normalization of head growth is associated with abnormal performance at 1 year and probably reflects significant white matter injury. Infants with surgically managed NEC have been shown to have significantly increased incidence of CP (24% versus 15%), deafness (4.1% versus 1.5%), and blindness (4.1% versus 1%).39 Meta-analysis of seven studies investigating the impact of NEC on neurodevelopmental outcome showed that infants with surgically treated NEC have a statistically significant increase in cognitive, psychomotor, and neurodevelopmental impairment compared with age-matched preterm infants without NEC.40 Impaired neurodevelopmental outcome in infants with NEC is further exacerbated by associated sepsis and the release of inflammatory cytokines and mediators in addition to hypoxia, all of which contribute to further insult to preoligodendrocytes, leading to white matter injury. The cerebellum is essential for gross and fine motor control, coordination, and motor sequencing and plays an important role in attention and language.36 The clinical hallmark of damage to the cerebellum is ataxia. However, recent advances in functional MRI (fMRI) have demonstrated that there are interactions between the cerebellum and nonmotor areas of the brain involved in language, attention, and mental imagery. Cerebellar injury can also be noted early in neonatal development from cranial ultrasound of the posterior fossa (mastoid view). The incidence of cerebellar injury may be as high as 20% in ELBW infants.41 Although the mechanism for damage is unknown, IVH is present in more than 75% of infants with cerebellar injury, implying similar risk factors for both IVH and cerebellar hemorrhage or the possibility that IVH leads to cerebellar hemorrhage. The majority of cerebellar lesions (70%) are unilateral. Preterm infants with isolated cerebellar hemorrhage exhibit significant neurological impairments: hypotonia (100%), abnormal gait (40%), ophthalmological abnormalities (approximately 40%), and microcephaly (17%).42 Overall, preterm infants with cerebellar hemorrhage performed significantly lower on tests of gross and fine motor skills and have deficits in vision and expressive and receptive language. Infants with both cerebellar injury and IVH have greater motor impairment than infants with isolated cerebellar hemorrhage. Socially, infants with isolated cerebellar hemorrhage exhibit delayed communication skills, decreased social skills with more withdrawn behavior, and impaired ability to attend to tasks. Thus, cerebellar injury increases the risk for poor neurodevelopmental outcome in cognition, learning, and behavior in preterm infants.42 For long-term effects of cerebellar damage, refer to Chapter 21. Perinatal asphyxia, the result of a hypoxic-ischemic (HI) insult, affects three to five per 1000 live births and leads to hypoxic-ischemic encephalopathy (HIE) in 0.5 to one per 1000 live births. Impaired oxygen delivery to the fetus can result from maternal hypotension, placental abruption, placental insufficiency, cord prolapse, prolonged labor, and/or traumatic delivery. Approximately 15% to 20% of infants with HIE will die, and 25% of the surviving infants will exhibit permanent neurological sequelae. Clinical findings will vary depending on the timing and duration of the HI insult, preconditioning and fetal adaptive mechanisms, comorbidities, and resuscitative efforts. Infants who are intrauterine growth restricted are at increased risk of an HI insult due to decreased nutrient reserves.43 It is important to note that the injury from an HI insult is an evolving and progressive process that begins at the time of the insult and continues through the recovery period (Figure 11-8). The HI insult causes decreased oxygen and glucose delivery to the brain, causing a shift from aerobic to anaerobic metabolism. This causes a decrease in adenosine triphosphate (ATP) production, leading to failure of the membrane-bound Na+-K+-ATPase pump. Sodium enters the neuronal cell, causing depolarization and release of excitatory neurotransmitters, specifically glutamate. This initial phase can last several hours and is marked by significant acidosis, depletion of high-energy compounds (energy failure), cellular swelling caused by entry of sodium and water, and cellular necrosis, causing spillage of intracellular contents into the extracellular space. The degree of neuronal necrosis is directly related to the duration and severity of the HI insult. During the subsequent reperfusion phase, free radical production increases and activation of microglia from extruded intracellular contents occurs, causing release of inflammatory mediators. A second phase of energy failure ensues, but without acidosis. Calcium enters the cell and the mitochondria, which then turns on the apoptotic pathway (programmed cell death). During this second phase of energy failure, seizures are often present. Activation of the apoptotic pathway accounts for the majority of cellular death and is the target for treatment.27,28 The specific timing of the initiation of the reperfusion phase and the second phase of energy failure is unclear in the clinical setting because the actual timing of the HI insult is not well defined. In animal studies, the latency between the first and second phases of energy failure is several hours. In term infants with HIE the cerebral damage is located in the deep structures of the brain (basal ganglia, thalamus, and posterior limb of the internal capsule) as well as the subcortical and parasagittal white matter.44 Diffusion-weighted MRI (DWI) is a very early diagnostic and sensitive technique to identify damage after the HI insult. As shown in Figure 11-8, a marked increase in signal in the subcortical and parasagittal white matter occurs as well as in the deep nuclear structures on DWI. MRI spectroscopy, localized to the basal ganglia or subcortical area, yields information about degree of secondary energy failure by analyzing for the depletion of the high-energy compound N-acetylaspartate and the presence of lactate.44,45 The degree of secondary energy failure as noted on MRI spectroscopy is predictive of death and poor neurodevelopmental outcome at 1 and 4 years of age. Classification of the clinical signs associated with HIE is shown in Table 11-3.46 Infants with grade 1 HIE rarely have long-term sequelae. Infants with grade 2 or moderate HIE have abnormal tone and reflexes and decreased spontaneous activity, with seizures commonly present. Approximately 10% of infants with moderate HIE will die and up to 30% will have neurodevelopmental delay. Infants with severe HIE (grade 3) exhibit minimal or no spontaneous activity or reflexes. Clinically evident seizures are seldom present, but electrographically evident seizures are more common. Approximately 50% of these infants die, and of the survivors, more than 60% to 80% are profoundly impaired. Long-term consequences of HIE include bulbar palsies with difficulties in sucking, swallowing, and facial movement. These infants have difficulty with secretions and may require tube feeding owing to inability to protect the airway. Upper-extremity involvement is more prominent than lower-extremity deficits because the damage to the cerebral cortex is located in the parasagittal region (see Figure 11-7). The development of epilepsy occurs in about 30% of infants with HIE. Mental retardation and difficulties at school age occur frequently. TABLE 11-3 SARNET SCORING SCALE FOR ENCEPHALOPATHY Modified from Sarnat HB, Sarnat MS: Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 33:696-705, 1976. Mild hypothermia (33.5° C) from application of cooling blankets or caps is becoming the standard of care for infants ≥ 36 weeks’ gestation who have an acute asphyxial event and moderate or severe HIE.47 Hypothermia has been shown to decrease cerebral metabolic demand and thus help preserve high-energy compounds. Hypothermia also delays membrane depolarization and decreases neuronal excitotoxicity. Free radical production and microglial activation are decreased. Most important, the activation of the apoptotic pathway is diminished. Transient side effects of hypothermia, such as bradycardia, mild hypotension, thrombocytopenia, and persistent pulmonary hypertension, can be medically treated and are usually not significant.48,49 A meta-analysis of published randomized studies comparing infants with moderate and severe HIE treated with either hypothermia or normothermia shows that hypothermic treatment significantly decreases mortality and morbidity (Table 11-4).50 Hypothermia appears to more efficacious in ameliorating brain damage in infants with mild HIE than in infants with severe HIE. It is known that hypothermia is most effective when administered before the onset of the second phase of energy failure. Because the HI insult can occur before delivery, it is postulated that hypothermia should be initiated as quickly as possible after delivery to increase the likelihood that it will diminish the neuronal damage and improve neurodevelopmental outcome. TABLE 11-4 EFFECT OF MODERATE HYPOTHERMIA ON NEUROLOGICAL OUTCOMES AT 18 MONTHS COMPARED WITH CONTROLS *Severe disability was defined in the CoolCap and TOBY trials as the presence of at least one of the following impairments: Mental Development Index score of less than 70 (2 standard deviations below the standardized mean of 100) on the Bayley Scales of Infant Development; gross motor function classification system level 3 to 5 (where the scale is from 1 to 5, with 1 being the mildest impairment); or bilateral cortical visual impairment with no useful vision. The NICHD trial defined disability as a Mental Developmental Index score of 70 to 84 plus one or more of the following impairments: gross motor function classification system level 2; hearing impairment with no amplification; or a persistent seizure disorder. †Survival with normal outcome was defined as survival without cerebral palsy and with a Mental Developmental Index score of more than 84, a Psychomotor Developmental Index score of more than 84, and normal vision and hearing. ‡Severe neuromotor delay was determined on the basis of a Psychomotor Developmental Index score of less than 70 in survivors. §Severe neurodevelopmental delay was determined on the basis of a Mental Developmental Index score of less than 70 in survivors. From Edwards AD, Brocklehurst P, Gunn AJ, et al: Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340:c363, 2010. The impact of maternal medications on the developing fetal brain depends on the specific drug as well as on the timing and duration of the drug exposure. Whereas the insults discussed previously cause predominantly cellular necrosis and apoptosis, medications given to the fetus and preterm infant cause alterations in the structure and function of genetic material as well as activation of the apoptotic pathway. The hypothesis that factors acting early in life have a long-lasting impact on development is called the Barker hypothesis or the fetal origins of adult disease. It is proposed that the biological value of this reprogramming is to prepare the fetus for maximal adaptation through methylation and deacetylation of histones, thereby determining the quantity of specific proteins that are produced. This topic is too extensive to be covered here and has been previously reviewed.51,52 This section will focus on heroin, methadone, cocaine, methamphetamine, and selective serotonin reuptake inhibitors (SSRIs) used in the treatment of maternal depression. It is difficult to ascertain the exact frequency of cocaine use during pregnancy, but reports indicate that 1% to 45% of females have used cocaine during their pregnancy.28 Cocaine is extracted from the leaves of the coca plant and can be smoked, inhaled, or injected into the bloodstream. Cocaine induces an intense and immediate euphoric state and can be very addictive. Unlike with opioids, physical dependence does not occur, but severe and intense cravings last for several months and can recur for years after cessation of cocaine use. Cocaine negatively affects neuronal proliferation, migration, growth, and connectivity, which distorts neuronal cortical architecture. However, the effects of intrauterine exposure to cocaine are difficult to determine because cocaine use is frequently associated with abuse of other illicit drugs, cigarettes, and alcohol. Other confounding variables include poor nutrition and limited prenatal care. In a large prospective blinded study, more infants exposed to cocaine in utero were delivered prematurely and exhibited decreased weight, length, and head circumference compared with matched controls.53 However, cocaine exposure did not affect the incidence of congenital abnormalities. The vasoconstrictive properties of cocaine increase the risk for HI injury and middle cerebral artery stroke. Neonates with prenatal cocaine exposure demonstrate tremors, hypertonia, irritability, and poor feeding ability. Cocaine-exposed infants have abnormal sleep patterns and are at a threefold to sevenfold increased risk of sudden infant death syndrome (SIDS). No difference was found in developmental testing54 between cocaine-exposed infants and matched controls, but the tests did not effectively evaluate arousal, emotional control, and social interaction.55 In utero cocaine exposure has been linked to increased incidence of behavioral problems and special education referrals in school-aged children. On fMRI, differences in the right frontal cortex and caudate nucleus are evident and indicate abnormalities in regulation of attention and cognitive abilities referred to as executive function. Opioid use during pregnancy has been associated with tubal pregnancies, premature rupture of membranes, uterine irritability, preterm labor, and preeclampsia. Infants exposed to opioids in utero are intrauterine growth retarded at birth, but methadone has a less severe impact on fetal growth. Infants exposed to opioids in utero are noted to have a decreased incidence of respiratory distress related to enhanced surfactant production56 and decreased hyperbilirubinemia as a result of induction of the enzyme glucuronyl transferase used in the metabolism of bilirubin. Withdrawal from narcotics occurs 2 to 3 days after delivery, but signs can be evident as long as 2 weeks after delivery. Methadone withdrawal usually occurs later than withdrawal from morphine or heroin related to its long half-life. Infants in withdrawal (neonatal abstinence syndrome [NAS]) exhibit gastrointestinal symptoms of vomiting and watery stools; neurological signs such as tremors; hypertonicity; high-pitched and incessant cry; hyperalert state; and sweating and fever. Infants with NAS have decreased ability to nipple feed despite excessive sucking on a pacifier. Seizures can be present in 2% to 11% of infants with NAS. Two commonly used scoring methods for severity of NAS are the Lipsitz57 and the Finnegan58 scales. The Lipsitz scale has 11 components that are scored from 0 to 3, with any score over 4 necessitating treatment.57 The Finnegan scale is a more comprehensive assessment, with more than 30 elements, and treatment is recommended if the score is greater than 8.58 A quiet, dimly lighted environment, decreased auditory stimulation, and swaddling or holding have been used to decrease neonatal irritability and pharmacotherapy. About 30% to 80% of in utero opioid-exposed infants will require medical treatment for NAS with morphine and either clonidine or phenobarbital. The goal of treatment is to decrease irritability, improve nippling efforts, and decrease vomiting and diarrhea. Infants exposed to opioids in utero continue to demonstrate tremulousness, hypertonicity, irritability, and increased crying episodes. In addition, they are less able to interact with people, demonstrate decreased age-appropriate free play, and have delayed fine motor coordination. The incidence of apnea and SIDS is increased in opioid-exposed infants. An appropriate and nurturing home environment is essential after discharge from the hospital to maximize neurodevelopmental outcome.59,60 SSRI medications such as fluoxetine (Prozac, Fontex, Seromex, Seronil), sertraline (Zoloft, Lustral, Serlain, Asenta), paroxetine (Paxil, Seroxat, Sereupin, Paroxat), fluvoxamine (Luvox, Favoxil), escitalopram (Lexapro, Cipralex, Esertia), and citalopram (Celexa, Seropram, Citox, Cital) are commonly prescribed to treat depression and anxiety disorders. The SSRI drugs inhibit serotonin reuptake, potentiating serotonergic neurotransmitter signaling. At least 600,000 infants are born yearly to mothers who have a major depressive disorder during their pregnancy.61 Medical therapy is the most common form of treatment for depression during pregnancy. Approximately 6% of pregnant woman use SSRIs during pregnancy, and almost 40% of depressed women have been reported to use antidepressants at some time during pregnancy.62 The serotonergic system is present early in gestation and is important in brain development. Perturbations in this system are associated with alterations in somatosensory processing and emotional responses. The SSRI medications readily cross the placenta and are linked to an increased risk of spontaneous abortion but not an increased incidence of malformations.61 A recently published meta-analysis found that maternal depression was significantly associated with an increased incidence of preterm labor and neonatal birth weight of less than 2500 g but not intrauterine growth retardation of the fetus.63 Unfortunately, this study was unable to evaluate the effect of SSRI therapy on these outcomes. Infants exposed to SSRIs in the third trimester have symptoms similar to withdrawal from opioid exposure (irritability, tremors, jitteriness, agitation, and difficulty sleeping). Neonatal feeding difficulties are common, and seizures and abnormal posturing are occasionally present. These symptoms are transient, appearing 2 to 4 days after birth and disappearing by the second week of life.64 It is difficult to identify any specific adverse neurodevelopmental outcomes in infants exposed prenatally to SSRIs from published studies because of the variability in the specific SSRI taken, the duration and timing of SSRI use, and the confounding factors of maternal depression and the use of multiple medications.65 Preterm births have increased over the last 10 years and now constitute about 13% of all births. Late preterm infants—that is, infants born at 34 to 366/7 weeks’ gestation—make up approximately 70% of preterm births.66 Many factors are implicated in the early delivery of late preterm infants, including preterm labor, preeclampsia, premature rupture of membranes, sepsis, and multiple gestation pregnancies. The late preterm infant is at increased risk of respiratory distress from insufficient surfactant production, transient tachypnea of the newborn from decreased pulmonary water absorption, persistent pulmonary hypertension, and complications of mechanical ventilation (pneumothorax). Hospital stay is prolonged in the late preterm infant compared with the infant born at term gestation owing to the increased difficulty with oral feeding, need for phototherapy for hyperbilirubinemia, and continuation of antibiotic therapy for suspected sepsis. Recent evidence has supported the concept that even healthy late preterm infants are at higher risk for neurodevelopmental delay compared with infants at term gestation. The late preterm infant’s brain is vulnerable to injury because a significant portion of brain development and maturation occurs during the last 2 months of pregnancy.67 Recent studies have shown that late preterm infants tested lower in reading skills in kindergarten and first grade, but not in math skills, than infants born at term gestation.68 However, kindergarten and first-grade teachers rated late preterm infants as not as competent as term infants in math and reading ability. Significantly more late preterm infants required special education in kindergarten and first grade compared with control infants. A trend toward increased enrollment in special education in the third and fourth grade was reported.68 Late preterm infants were considered at increased risk for (1) developmental delay at 3 and 4 years of age, (2) retention in kindergarten, and (3) referral for special education.69 However, maternal age and education were significantly decreased in late preterm infants compared with infants born at term gestation. In addition, the use of Medicaid, insufficient medical care, and maternal tobacco use were higher in the mothers of late preterm infants. These multiple factors, as well as the home environment, are significant risk factors in determining the effects of late preterm delivery on long-term outcome. Regardless of the specific insult, late preterm infants are at increased risk of neurodevelopmental disabilities and should receive timely developmental follow-up to identify potential underachievement and behavioral problems.
Neonates and parents: neurodevelopmental perspectives in the neonatal intensive care unit and follow-up
Theoretical framework
Dynamic systems
Synactive model of infant behavior
Hope-empowerment model
Neonatal complications associated with adverse outcomes

AGE
SPECIFIC INSULT
Preterm
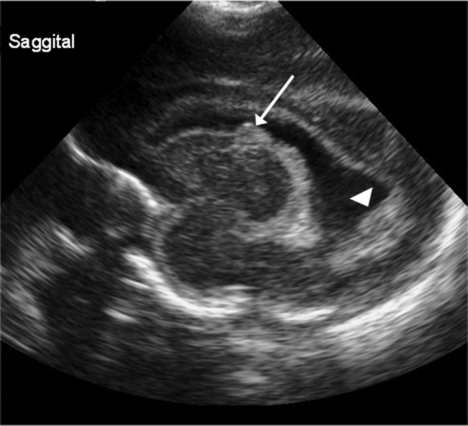
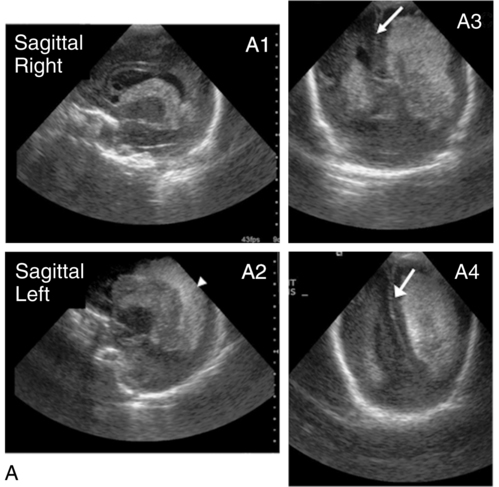
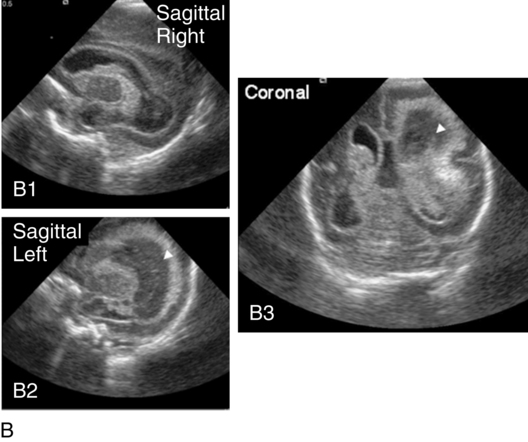
Intraventricular hemorrhage (IVH)Periventricular hemorrhagic infarct (PVHI)
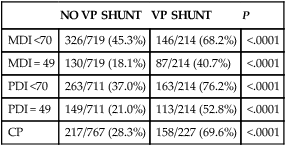
Posthemorrhagic ventricular dilatation (PHVD)
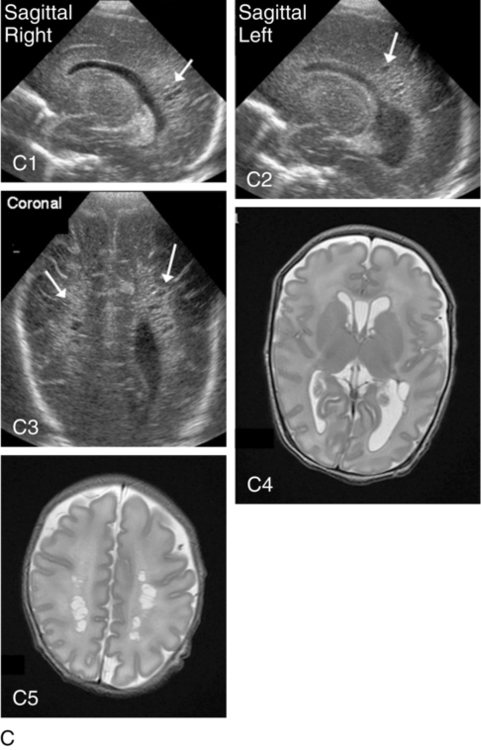
Periventricular leukomalacia (PVL)
Necrotizing enterocolitis (NEC)
Term
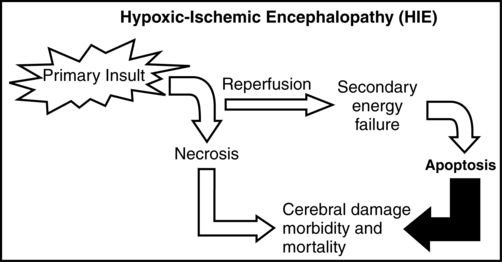
Hypoxic-ischemic encephalopathy (HIE)
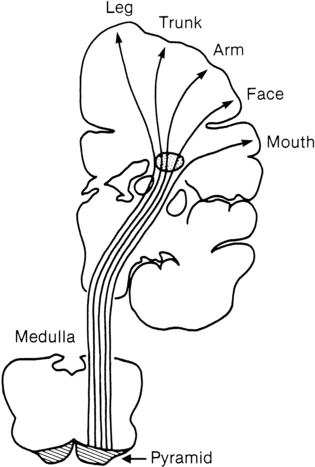
Intraventricular hemorrhage
Periventricular hemorrhagic infarct
Posthemorrhagic ventricular dilatation

NO VP SHUNT
VP SHUNT
P
MDI <70
326/719 (45.3%)
146/214 (68.2%)
<.0001
MDI = 49
130/719 (18.1%)
87/214 (40.7%)
<.0001
PDI <70
263/711 (37.0%)
163/214 (76.2%)
<.0001
PDI = 49
149/711 (21.0%)
113/214 (52.8%)
<.0001
CP
217/767 (28.3%)
158/227 (69.6%)
<.0001
Periventricular leukomalacia
Necrotizing enterocolitis
Cerebellar injury
Hypoxic-ischemic encephalopathy

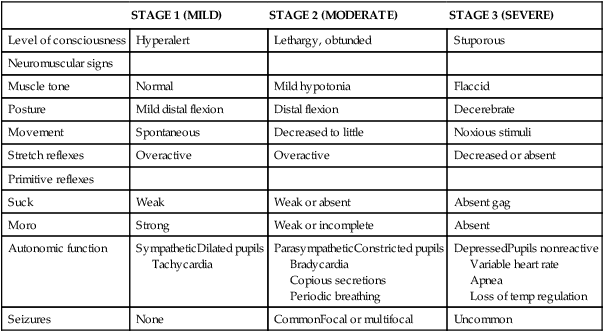
STAGE 1 (MILD)
STAGE 2 (MODERATE)
STAGE 3 (SEVERE)
Level of consciousness
Hyperalert
Lethargy, obtunded
Stuporous
Neuromuscular signs
Muscle tone
Normal
Mild hypotonia
Flaccid
Posture
Mild distal flexion
Distal flexion
Decerebrate
Movement
Spontaneous
Decreased to little
Noxious stimuli
Stretch reflexes
Overactive
Overactive
Decreased or absent
Primitive reflexes
Suck
Weak
Weak or absent
Absent gag
Moro
Strong
Weak or incomplete
Absent
Autonomic function
SympatheticDilated pupils
Tachycardia
ParasympatheticConstricted pupils
Bradycardia
Copious secretions
Periodic breathing
DepressedPupils nonreactive
Variable heart rate
Apnea
Loss of temp regulation
Seizures
None
CommonFocal or multifocal
Uncommon

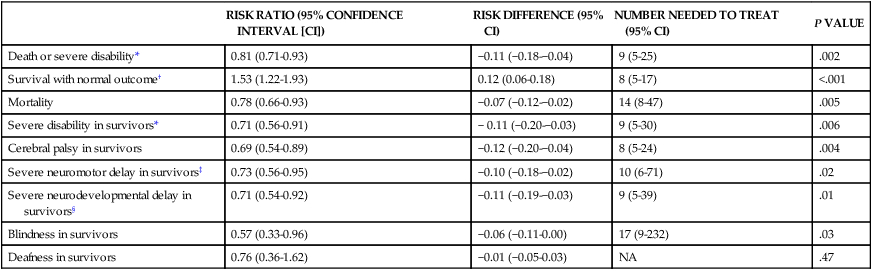
RISK RATIO (95% CONFIDENCE INTERVAL [CI])
RISK DIFFERENCE (95% CI)
NUMBER NEEDED TO TREAT (95% CI)
P VALUE
Death or severe disability*
0.81 (0.71-0.93)
−0.11 (−0.18-−0.04)
9 (5-25)
.002
Survival with normal outcome†
1.53 (1.22-1.93)
0.12 (0.06-0.18)
8 (5-17)
<.001
Mortality
0.78 (0.66-0.93)
−0.07 (−0.12-−0.02)
14 (8-47)
.005
Severe disability in survivors*
0.71 (0.56-0.91)
− 0.11 (−0.20-−0.03)
9 (5-30)
.006
Cerebral palsy in survivors
0.69 (0.54-0.89)
−0.12 (−0.20-−0.04)
8 (5-24)
.004
Severe neuromotor delay in survivors‡
0.73 (0.56-0.95)
−0.10 (−0.18-−0.02)
10 (6-71)
.02
Severe neurodevelopmental delay in survivors§
0.71 (0.54-0.92)
−0.11 (−0.19-−0.03)
9 (5-39)
.01
Blindness in survivors
0.57 (0.33-0.96)
−0.06 (−0.11-0.00)
17 (9-232)
.03
Deafness in survivors
0.76 (0.36-1.62)
−0.01 (−0.05-0.03)
NA
.47
Maternal medication
Cocaine
Opioids
Selective serotonin reuptake inhibitors
Late preterm birth
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree