12 Transcutaneous electrical nerve stimulators for pain management
CHAPTER CONTENTS
Introduction
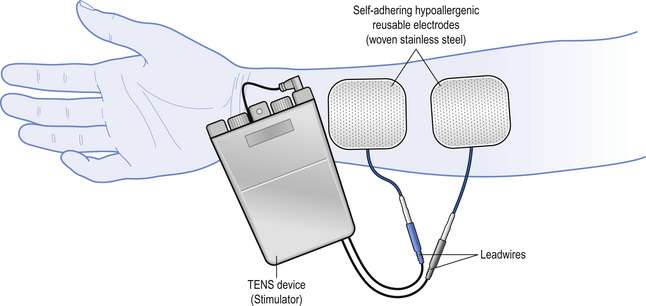
Transcutaneous electrical nerve stimulation (TENS) is a peripheral stimulation technique that is non-invasive, allowing patients the ability to self-administer treatment. The purpose of TENS is to deliver pulsed electrical currents across the intact surface of the skin to activate underlying nerves and reduce pain (Fig. 12.1). Effective treatment is facilitated when administered to produce a strong non-painful electrical paraesthesia. The effects are usually rapid in onset and offset, allowing treatment administration throughout the day. TENS is inexpensive and can be purchased without prescription in the UK. However, a practitioner who has been trained in the principles and practice of TENS should supervise patient’s use in the first instance and provide a point of contact to troubleshoot any problems.
Electrotherapy became popular in the eighteenth and nineteenth centuries following the invention of electrostatic generators. However, increasing use of pharmacological treatments in the twentieth century meant that electrotherapy disappeared from mainstream medicine until the mid-1960s. Interest in electrotherapy for pain relief increased with the publication of Melzack and Wall’s Pain Mechanisms: A New Theory (Melzack & Wall 1965). They suggested that large diameter non-noxious transmitting peripheral afferents could be stimulated using electrical stimuli, reducing onward transmission of noxious information arising from tissue damage. In 1967 Wall & Sweet reported that electrical stimulation of peripheral nerves reduced pain in eight chronic pain patients (Wall & Sweet 1967). Pain relief was also demonstrated in patients during electrical stimulation of dorsal columns (Shealy et al 1967) and the periaqueductal grey of the midbrain, forming part of the descending pain inhibitory pathways (Richardson & Akil 1977). Originally, TENS was used to predict the success of dorsal column stimulation implants until it was realized that it could be used as a successful modality on its own (Long 1973; Shealy 1972).
Definition and techniques
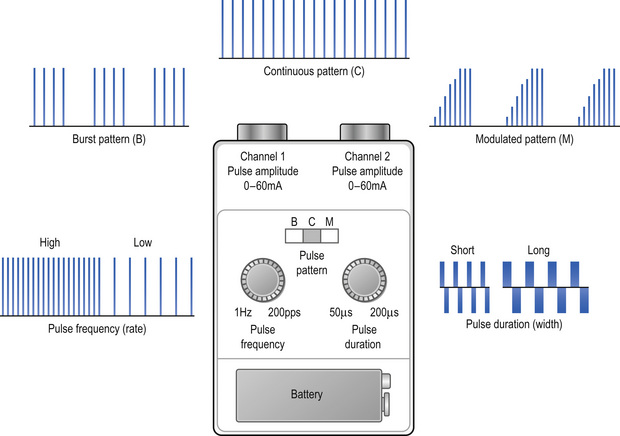
Healthcare professionals use the term TENS to refer to currents administered using a ‘standard TENS device’ (Fig. 12.2). Differences in the design between manufacturers tend to be cosmetic with limited effect on physiological and clinical outcome. Some manufacturers have designed TENS devices that markedly differ from a standard device. These TENS-like devices include interferential therapy, microcurrent therapy, and transcutaneous electrical acupoint stimulation. A critical review of TENS-like devices can be found in Johnson (2001a, b). A standard TENS device should be used for pain in the first instance and will be the focus of this chapter.
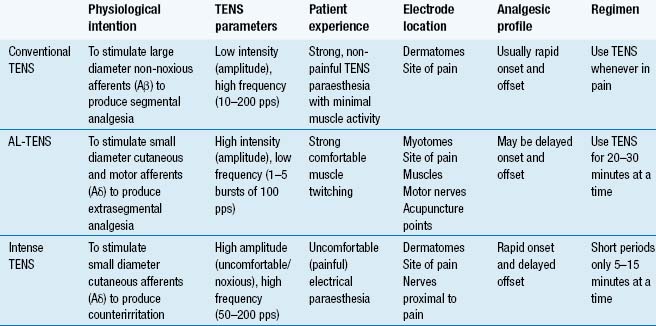
The purpose of TENS is to stimulate nerve fibres and to generate nerve impulses that elicit pain modulation. Different techniques are used to stimulate different populations of nerve fibres (Table 12.1). The main techniques are:
Conventional TENS is used for most patients in the first instance.
Conventional TENS
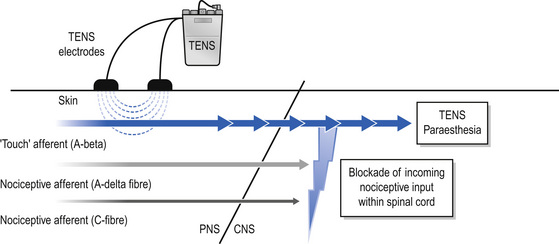
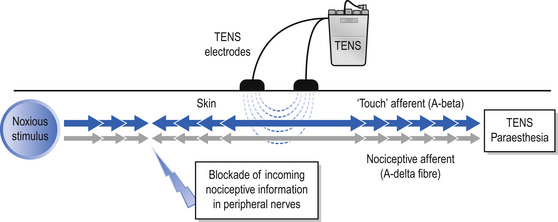
The International Association for the Study of Pain (IASP) defines conventional TENS as high frequency (50–100 Hz), low intensity (paraesthesia, not painful), small pulse width (50–200 μs) (Charlton 2005). Conventional TENS is used to activate low-threshold, large diameter myelinated afferent fibres (Aβ) normally transmitting information related to non-painful touch and pressure (Fig. 12.3). This inhibits onward transmission of nociceptive information at synapses in the central nervous system (see Mechanism of Action). Patients are instructed to increase TENS pulse amplitude until a strong, comfortable, non-painful paraesthesia is experienced beneath the electrodes, indicating large diameter myelinated afferent fibre activity. A painful TENS paraesthesia beneath the electrodes is not appropriate. Theoretically, high-frequency (∼10–200 pulses per second (pps)) currents are optimal because they generate a large afferent barrage leading to greater inhibition of nociceptive transmission. Pulse durations between 50 and 200 μs allow optimal precision in achieving the desired intensity when titrating pulse amplitude.
Acupuncture-like TENS (AL-TENS)
AL-TENS was developed to harness the mechanisms of action of TENS and acupuncture by activating segmental and extrasegmental mechanisms (descending pain inhibitory pathways) (Eriksson & Sjölund 1976). IASP define AL-TENS as a form of hyperstimulation achieved using currents that are low frequency (2–4 Hz), higher intensity (to tolerance threshold), and longer pulse width (100–400 μs) (Charlton 2005). Intermittent trains or bursts (2–4 Hz) of high-frequency pulses (100–200 pps) are often used in clinical practice to reduce discomfort experienced using high-intensity single pulses. The intention of AL-TENS is to stimulate small diameter, higher threshold afferents (Aδ) using high-intensity, low-frequency TENS. Research suggests that small muscle afferents produce greatest analgesia so some practitioners administer AL-TENS to generate non-painful muscle twitches which indirectly generates impulses in small diameter muscle afferents (Fig. 12.4). Electrodes are positioned at the site of pain, over myotomes, muscles, acupuncture points, and trigger points. AL-TENS is used to treat patients who are resistant to conventional TENS and patients are advised to administer it less frequently than conventional TENS, e.g. 20 minutes, 3 times a day (Eriksson & Sjölund 1976). AL-TENS can also be used for muscle and visceral pain arising from deep-seated structures, radiating neuropathic pain, and in situations where prolonged analgesia is required (Johnson 1998).
Intense TENS
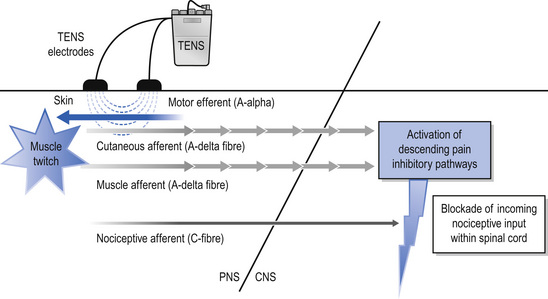
Intense TENS is a counterirritant and is delivered for short periods of time over nerve bundles close to the site of pain. High-frequency (up to 200 pps), high-intensity currents that are painful but tolerable are used. The intention of intense TENS is to stimulate small diameter, higher threshold cutaneous afferents (Aδ) to block transmission of nociceptive information in peripheral nerves (Fig. 12.5). Intense TENS activates diffuse noxious inhibitory controls (Le Bars et al 1979), and can be used for minor procedures such as wound dressing and suture removal.
Contraindications
Manufacturers list cardiac pacemakers, epilepsy, and pregnancy as contraindications because it may be difficult to exclude TENS as a potential cause from a medico-legal perspective. The Chartered Society for Physiotherapy (CSP) suggest that TENS can be used in pregnancy and in epilepsy providing electrodes are placed well away from the abdomen, sacrum, and neck respectively (i.e. local contraindication) (CSP 2006). The CSP also lists bleeding tissue as a contraindication and suggests that TENS should not be delivered over active epiphysis or over an active, treatable tumour.
Precautions
TENS should not be administered over the anterior neck, eyes, and testes or through the chest using anterior and posterior positions. TENS may interfere with foetal and cardiac monitoring equipment and should not be administered close to transdermal drug delivery systems. There is no known evidence that adverse events occur when TENS is used with metal implants, stents, percutaneous central catheters, or drainage systems. It should not be used while driving and should only be given internally using devices designed for that purpose (e.g. incontinence or dental analgesia). TENS devices with timers that automatically switch off are useful to aid sleep and may be used by children with success (Lander & Fowler-Kerry 1993; Merkel et al 1999).
Serious adverse events from TENS occur but are extremely rare (Mann 1996; Rosted 2001). It has been known to exacerbate pain and occasionally causes nausea and light-headedness, but retains an excellent safety and toxicity profile. No major drug interactions occur; therefore it can be combined with analgesics to reduce dosage and drug-related side effects. It has been claimed that caffeine may inhibit TENS effects (Marchand et al 1995).
Clinical technique
Indications
TENS is potentially useful for any type of pain including that of nociceptive, neuropathic, and musculoskeletal origins (Table 12.2). Clinical experience suggests it provides long-term benefit for low back pain (LBP), osteoarthritis (OA), localized muscle pain, and neuropathic pains of peripheral origin such as postherpetic and trigeminal neuralgias, amputee pain, entrapment neuropathies, and radiculopathies (Barlas & Lundeberg 2006). TENS may also benefit metastatic bone disease, nerve compression by a neoplasm, and post-mastectomy and post-thoracotomy pains (Berkovitch & Waller 2005).
Table 12.2 Clinical Indications
| Pain | Chronic pain |
|---|---|
| Postoperative pain | Osteoarthritis, rheumatoid arthritis, low back pain |
| Labour pain | Neuropathic pain including amputee pain, postherpetic and trigeminal neuralgias, post-stroke pain, complex regional pain syndrome |
| Dysmenorrhoea | Localized muscle pain including muscle tension, myofascial pain, post-exercise soreness |
| Angina pectoris | Nociceptive pain including inflammatory pains and chronic wound pain |
| Orofacial pain | Cancer-related pain |
| Physical trauma including fractured ribs and minor medical procedures | Acute pain |
Electrode location
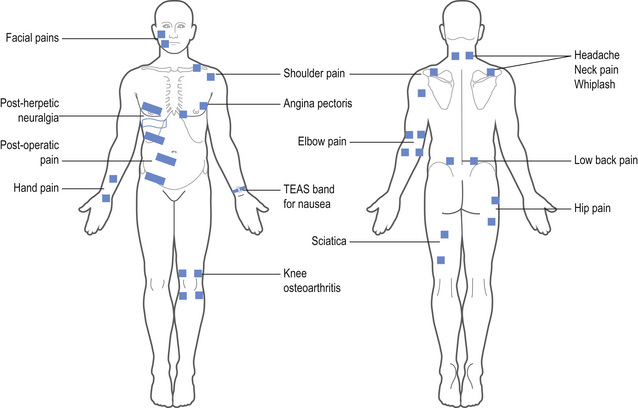
TENS should be delivered over healthy sensate skin; therefore skin sensitivity testing should be undertaken at the site of electrode placement. Electrodes are positioned at dermatomes related to the site of pain for conventional TENS. As TENS activates nerve fibres directly beneath the electrodes the primary site for electrodes is around the site of pain (Fig. 12.6), or positioned paravertebrally at the appropriate spinal segment or on contralateral dermatomes. If it is not possible to site electrodes close to the pain because of hypersensitivity or skin damage (e.g. open wound, eczema), then electrodes should be positioned on nerves proximal to the pain. TENS may aggravate pain if electrodes are placed on skin with tactile allodynia.
TENS on acupuncture points
The use of TENS to supplement acupuncture analgesia over specific points, such as trigger and acupuncture points, is done sparingly within clinical application. A common misconception is that AL-TENS must be delivered at acupuncture points, which is not the case, but it may be effective. A review of research on TENS and acupuncture points concluded that it may be useful when given over acupuncture points but there were few studies that compared TENS at acupuncture points versus TENS at the site of pain (Walsh 1996).
Transcutaneous electrical acupoint stimulators (TEAS) are watch-like devices worn on the underside of the wrist over the Pericardium 6 (P6) acupuncture point (Fig. 12.6). Good quality randomized controlled trials (RCTs) have found that TEAS reduced postoperative and chemotherapy-induced nausea and vomiting (Coloma et al 2002; Zarate et al 2001).
Electrical characteristics of TENS
The key determinant of TENS outcome is titration of the pulse amplitude to activate different nerve fibres (Table 12.1). For conventional TENS the user should titrate pulse amplitude to produce a strong, comfortable, non-painful paraesthesia beneath the electrodes. Practitioners should be cautious of claims about the best pulse frequencies, durations, and patterns for different pain conditions. A systematic review of studies investigating the effects of different pulse frequencies on experimental pain in healthy humans concluded that research to find optimal TENS settings for different conditions is confusing (Chen et al 2008) suggesting that the parameters may influence subjective comfort of paraesthesia rather than having clinically meaningful effects on TENS outcome (Johnson et al 1991a, b). For this reason, pulse frequency, pattern, and duration are selected by trial and error according to ‘personal comfort’ for the pain at that time. Patients are encouraged to experiment with settings within and between treatments whilst maintaining a strong but comfortable intensity.
Research evidence
Mechanism of action
TENS causes antridromic activation of peripheral nerves so that impulses travelling away from the central nervous system will collide and extinguish afferent impulses arising from peripheral receptors. This may lead to peripheral blockade of impulses arising from tissue damage (Fig. 12.5).
Animal studies show that conventional TENS inhibits central transmission of nociceptive information in the spinal cord when applied to somatic receptive fields (Garrison & Foreman 1994, 1996; Leem et al 1995). The inhibitory neurotransmitter gamma-amino butyric acid (GABA) appears to be critical for conventional TENS effects (Duggan & Foong 1985; Maeda et al 2007). It has also been shown to reduce inflammation-induced sensitization of dorsal horn neurons in anaesthetized rats (Ma & Sluka 2001).
Higher intensities, e.g. AL-TENS, act via extrasegmental mechanisms and activate structures on the descending pain inhibitory pathways (e.g. periaqueductal grey and ventromedial medulla) and inhibit structures on descending pain facilitatory pathways (Ainsworth et al 2006; Chung et al 1984a, b). Higher intensities cause long-term depression of central nociceptor cells for up to 2 hours post stimulation (Sandkühler et al 1997, 2000). Activation of deep tissue peripheral afferents appears to produce largest effects (Duranti et al 1988; Radhakrishnan & Sluka 2005). Brief, intense, painful TENS probably elicits counterirritant mechanisms via diffuse noxious inhibitory controls (Le Bars et al 1979).
Recent research has shown low-frequency TENS to involve mu opioid receptors and high-frequency TENS to involve delta opioid receptors (Kalra et al 2001; Sluka et al 1999, 2000). Cholinergic, adrenergic, and serotinergic systems also seem to be involved (King et al 2005; Radhakrishnan et al 2003; Sluka & Chandran 2002).
Clinical effectiveness
There are over 500 RCTs cited in PubMed (10 September 2009) but many have methodological shortcomings due to inappropriate technique and/or under dosing. Systematic reviews of clinical research for acute pain have been inconclusive for a mix of acute pain conditions (Walsh et al 2009), positive for primary dysmenorrhoea (Proctor et al 2003) and negative for labour pain (Carroll et al 1997; Dowswell et al 2009) and postoperative pain (Carroll et al 1996). However, a systematic review of 21 RCTs on TENS for postoperative pain revealed shortcomings in RCTs that may have contributed to negative findings (Bjordal et al 2003). The meta-analysis demonstrated TENS reduced analgesic consumption during postoperative care, provided it was administered using a strong, subnoxious electrical stimulation at the site of pain.
Systematic reviews for chronic pain are often inconclusive (Nnoaham and Kumbang 2008; Khadilkar et al 2005) although authors are often positive about TENS effects. It may be of benefit for, knee OA (Osiri et al 2000; Bjordal et al 2008), rheumatoid arthritis of the hand (Brosseau et al 2003), post-stroke shoulder pain (Price & Pandyan 2000), whiplash, mechanical neck disorders (Kroeling et al 2005), and chronic recurrent headache (Bronfort et al 2004). a meta-analysis of 38 studies on TENS and peripheral electrical nerve stimulation (PENS) for chronic musculoskeletal pain reported significant decreases in pain at rest and on movement (Johnson & Martinson 2007). There is insufficient evidence to judge the effects of TENS for cancer pain (Robb et al 2009)
Introduction
Complex regional pain syndrome type 1 (CRPS 1) was previously classified as reflex sympathetic dystrophy (RSD) (Evans 1946) and refers to a functional disorder of the spinal cord that involves the dorsal and ventral horns, and the intermediolateral columns, to varying degrees so as to produce sensory, motor, and autonomic abnormalities (Loeser 2005; Wilson et al 2005a). Type I CRPS is distinguished from type II solely by the presence or absence of a clinically detectable injury or nerve involvement. The condition is a form of neuropathic pain, but not all neuropathic pain are caused by CRPS and not all neuropathies lead to presentations of this type (Loeser 2005). The symptoms of CRPS 1 may be caused by an injury or by spontaneous events, manifesting via pain and sensory changes disproportionate in intensity, distribution, and duration to the underlying pathology (Dunn 2000). Additional dysfunctional features may involve motor changes, autonomic changes, trophic changes, and psychological dysfunction.
CRPS 1 is now regarded as a systemic condition involving the entire neuroaxis with manifestations of inflammatory changes at the central and peripheral nerve levels. It is a syndrome that represents a spectrum of changes involving a myriad of multiple systems including neurogenic both peripheral (PNS) and central nervous systems (CNS); endocrine; vascular; musculoskeletal; and biopsychosocial (Wilson et al 2005b). The condition appears to have a cyclical presentation, with recurrences of symptoms after dormant periods ranging from 6 months to 2 years; recurrent episodes are reported as occurring in 10 to 30% of patients diagnosed with the condition (Dunn 2000).
Current evidence is far from conclusive and a wide variety of causative mechanisms have been described (van Griensven 2005), with generalized sensory and motor changes not explained by the peripheral innervation (Rommel et al 1999) and even altered brain responses (Juottonen et al 2002). There appears to be no evidence of CRPS as a psychogenic condition, merely anxiety and stress linked to the physical presentation alongside sympathetic dysfunction (Covington 1996
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Case Study 1
Case Study 1





