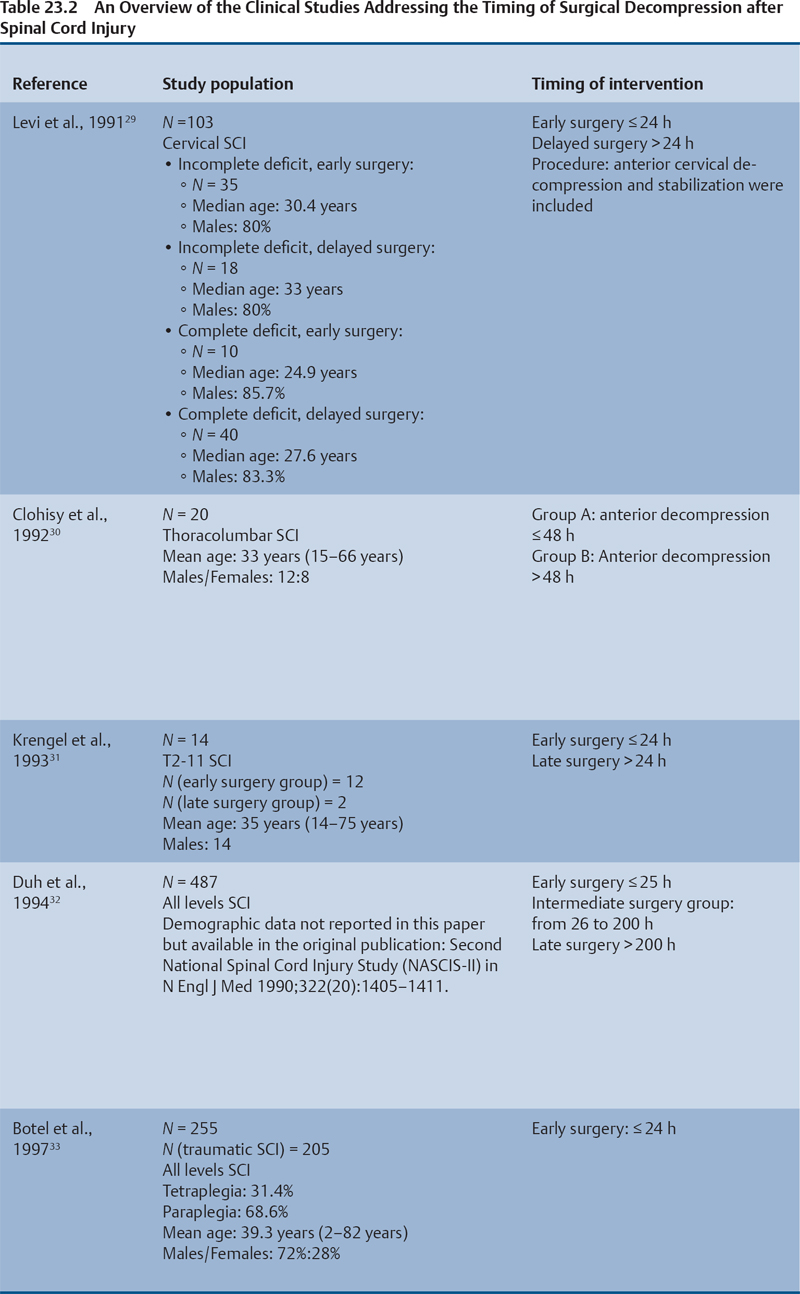
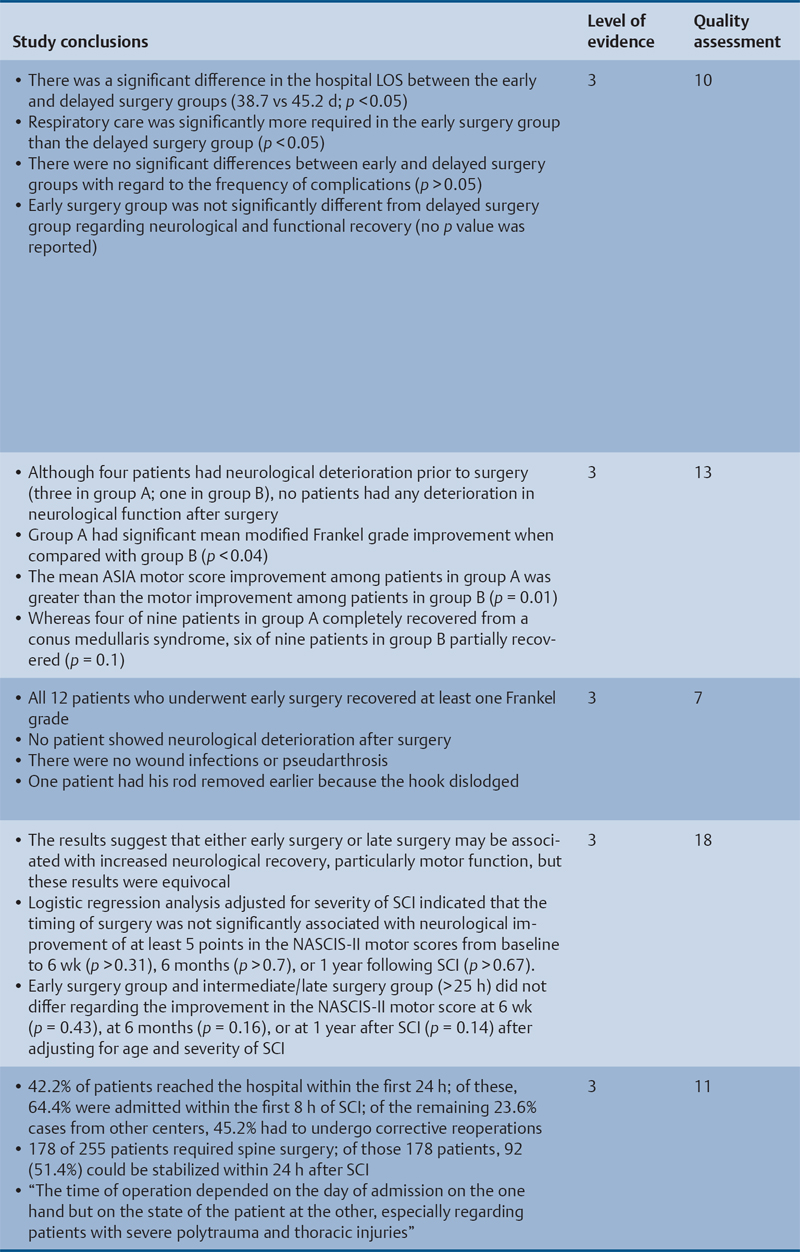
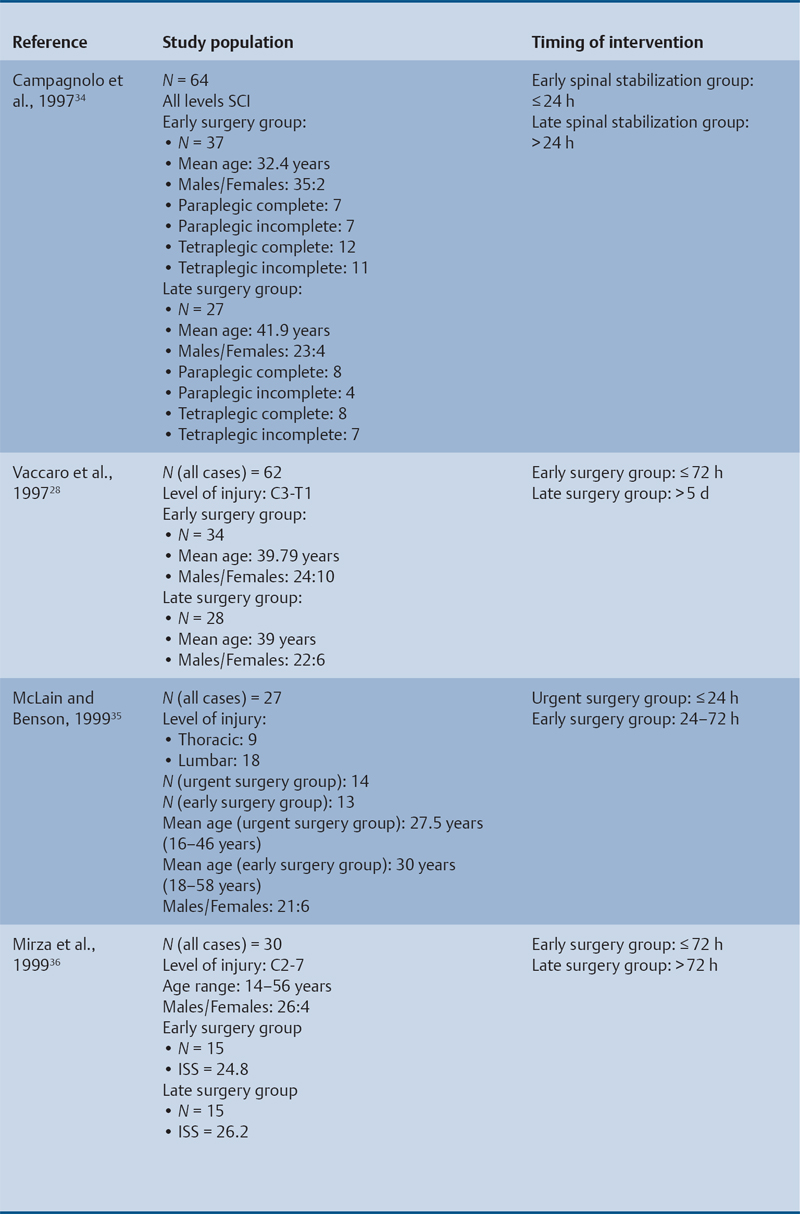
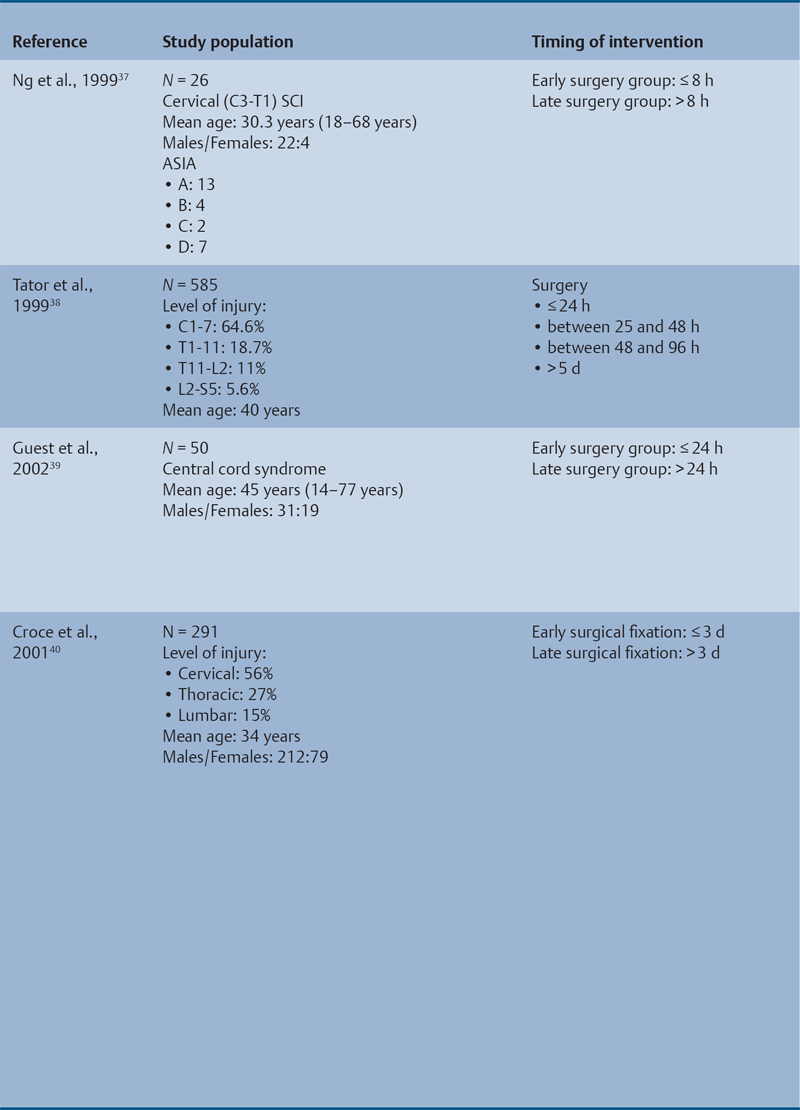
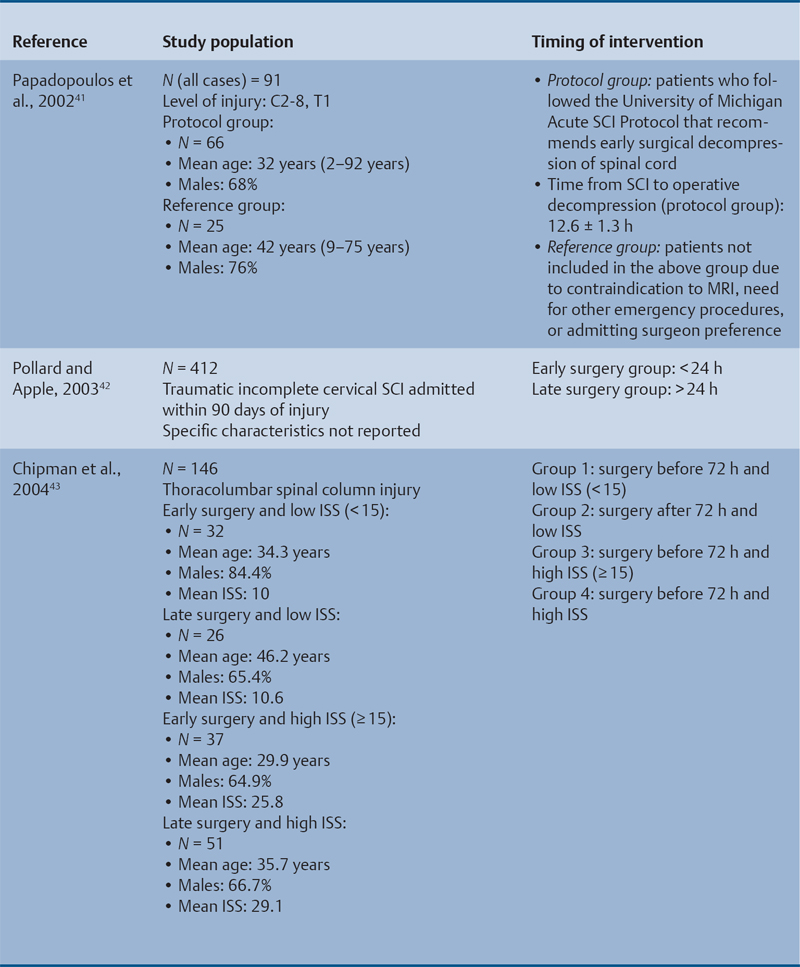
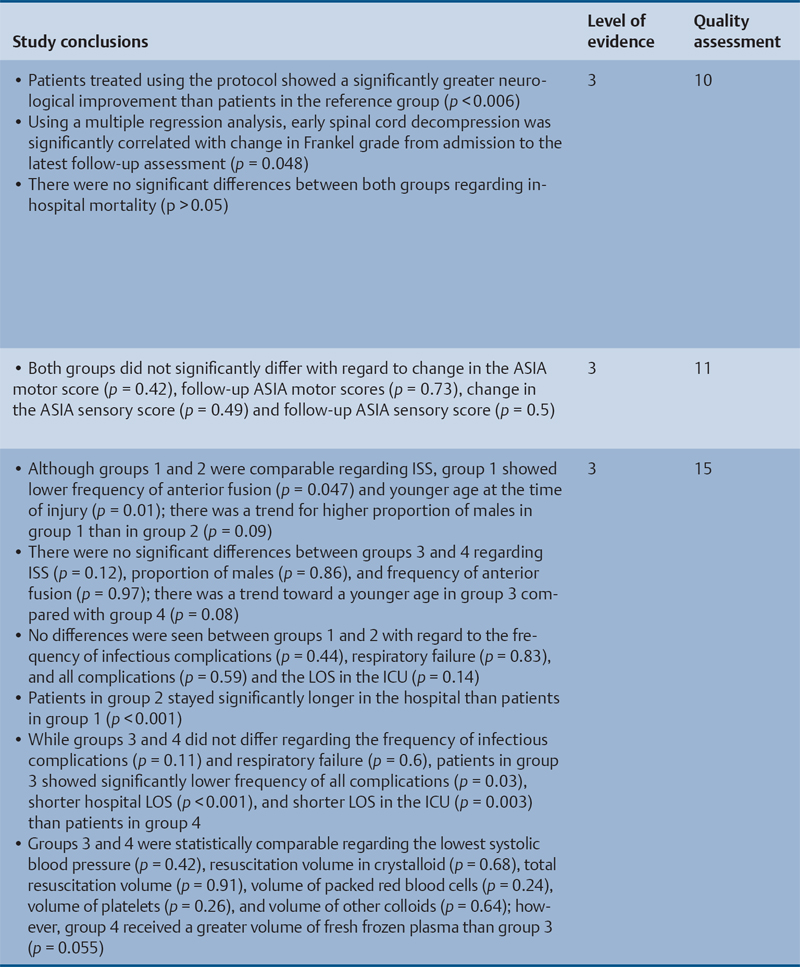
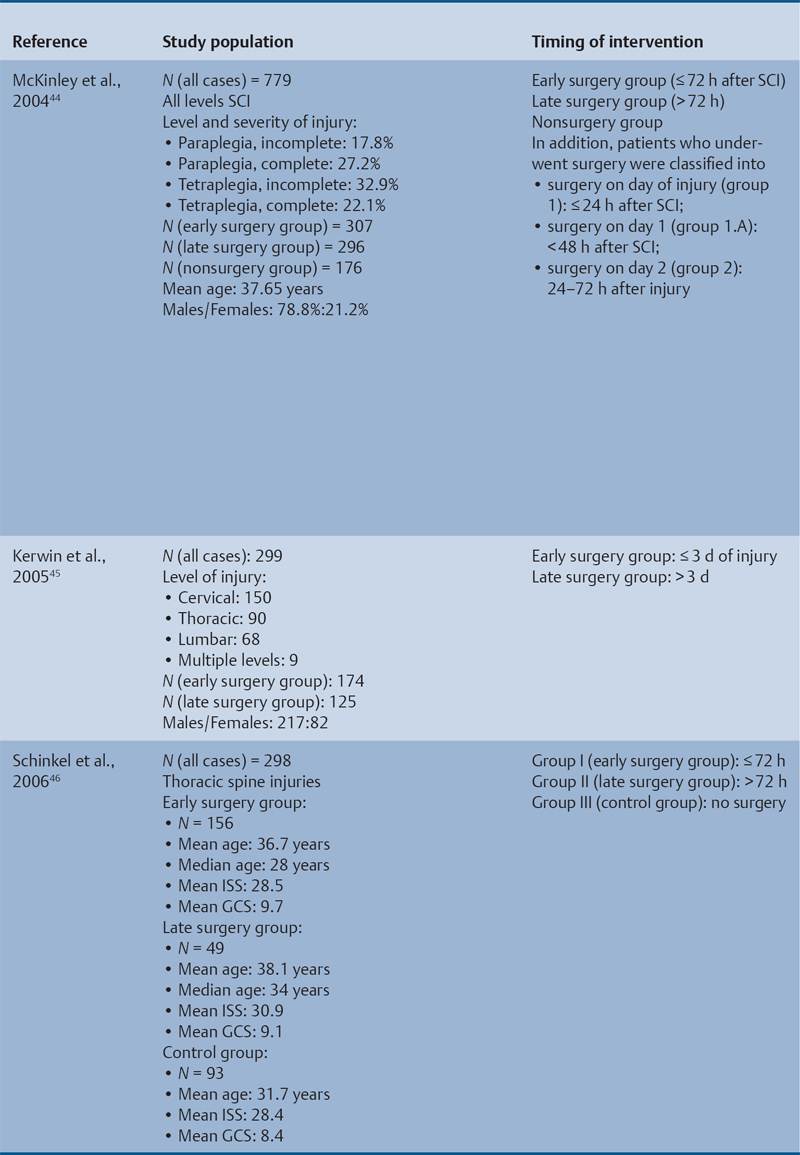
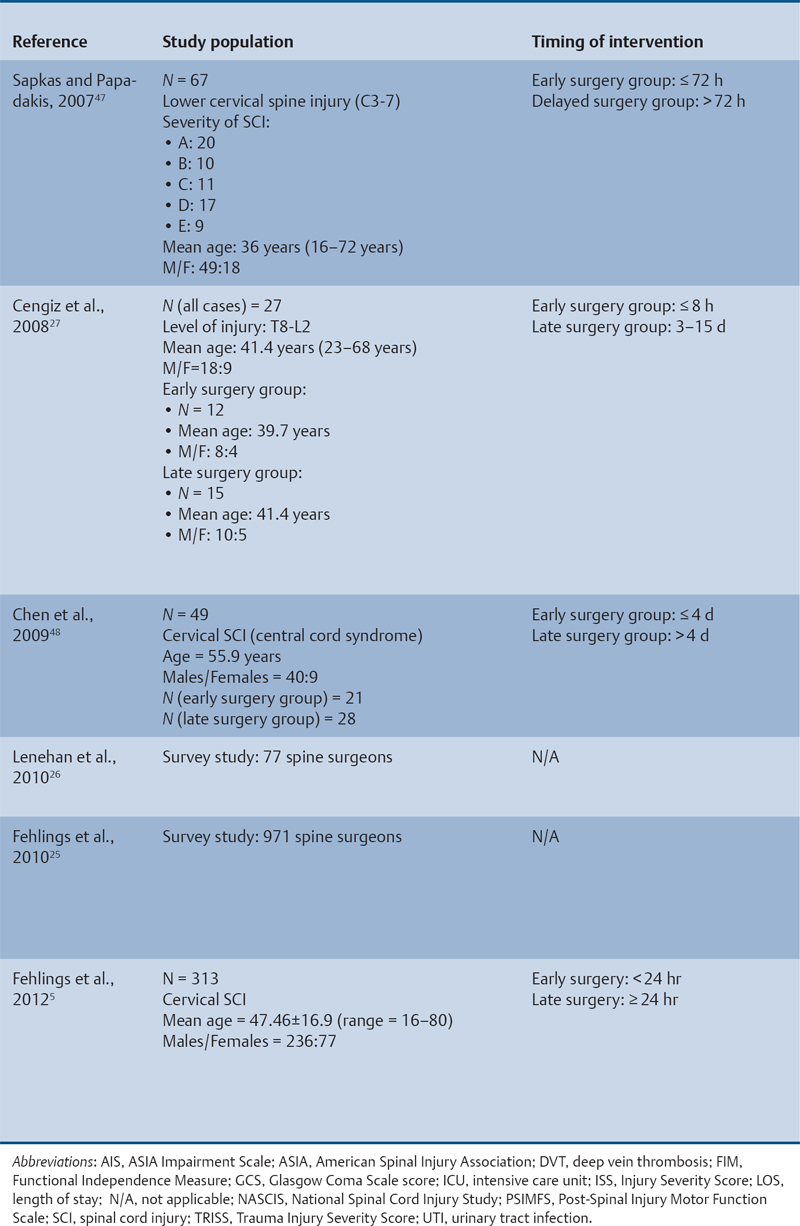
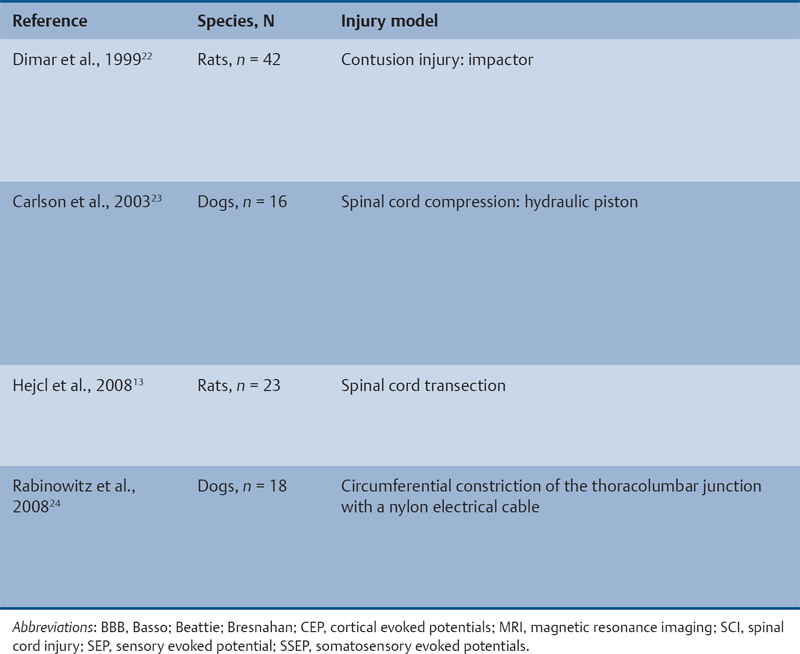
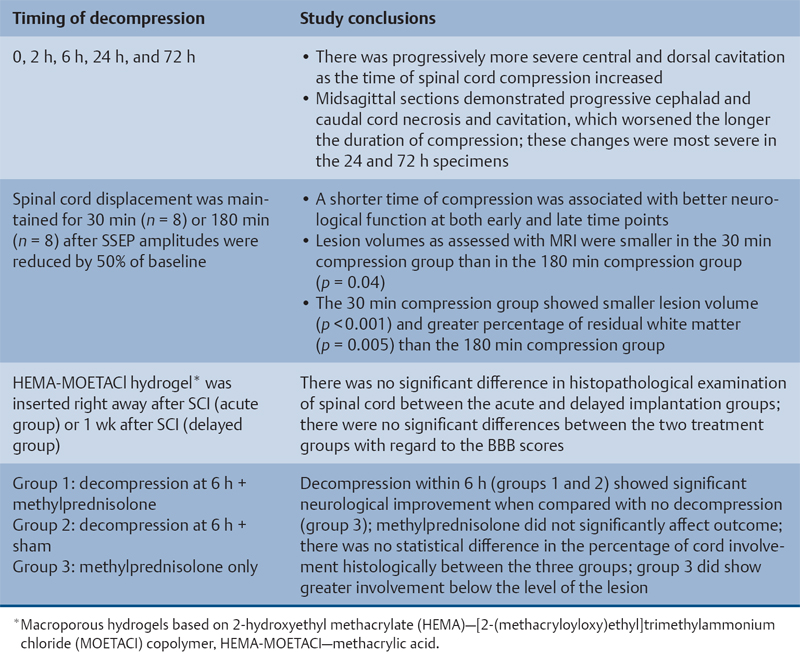
23 Key Points 1. A systematic review was conducted to evaluate the evidence relating to the timing of decompression for spinal cord injury. 2. Although animal evidence largely supports early decompression after spinal cord injury, the preclinical and clinical evidence in humans is less clear. 3. Early decompression—within 24 hours of injury—should be strongly considered as an option for patients who are medically stable without major comorbidities or multiple traumas Spinal cord injury (SCI) is a devastating condition with immense physical, emotional, and economic costs for the patient, family, and health care system. Treatments that can increase recovery and decrease complications could address these issues. To this end, the rate of discovery in basic, translational, and clinical research in neurotrauma has been increasing over many years, particularly with regard to the pathophysiology of traumatic SCI. The focus of treatment strategies for SCI has aimed to minimize the deleterious effects of secondary injury and to lead to an environment conducive to recovery. Several such translational strategies have either made their way into clinical practice or are the focus of ongoing clinical trials to determine their safety and efficacy in patients with SCI. Despite this progress, pharmacological therapies have yet to affect outcomes in a predictable and consistent fashion. Furthermore, the current standard of care for a patient with a new spinal cord injury varies from hospital to hospital. Surgical decompression, however, is a treatment option that aims to reduce neurological deficit occurring as a result of secondary damage and is currently widely practiced. Persistent compressive forces from the injured spinal column contribute to ongoing secondary damage. This issue leads to the theory behind decompressive surgery, that removing compression will reduce secondary damage. There may be several indications for surgery following traumatic SCI. Although there is little controversy over the need for surgical stabilization for spinal instability caused by torn ligaments and bony fracture, there remain some questions about surgical decompression that aims to improve neurological outcome without a strong indication for the treatment of spinal column instability. This chapter discusses this issue. As already mentioned, physical compression of the spinal cord is responsible for triggering an ongoing series of deleterious cascades; surgical decompression aims to relieve this compression. Evidence is also mounting for improved outcomes with decompressive surgery and the timing threshold associated with these improved outcomes. Recent systematic reviews of the literature suggest that there is a biological basis for surgical decompression of the spinal cord within 24 hours of injury.1–4 Furthermore, results from the Surgical Treatment of Acute Spinal Cord Injury Study (STASCIS) indicate that decompression within 24 hours of injury improves outcome in patients with isolated SCI.5 This chapter reviews the current evidence for early decompression. Given the background information presented earlier, a knowledge gap was identified that has been addressed by several preclinical and clinical research studies. The results of these studies are varied and focus on many different aspects of surgical treatment for SCI. Two questions were developed to frame the literature search and address this aim: 1. Do preclinical studies confirm the biological basis for surgical decompression after SCI? 2. What are the neurological and functional outcome effects of early surgical decompression in the clinical setting? The MEDLINE database (1950–May 2010) and the EMBASE database (1980– 2010) were searched. The search terms were “timing” AND “decompression” AND “spinal cord injury.” This search strategy revealed 66 results. After application of the inclusion and exclusion criteria to eliminate irrelevant articles, 38 studies remained for analysis. All original research papers in the English language were included. All clinical case reports, in vitro experiments, photochemically induced injury models, and nerve root or peripheral nervous system injury models were excluded. Lastly, review articles were read and the references hand searched to ensure that all relevant studies were captured, but the review papers themselves were not included in the results. Review articles provided an additional three articles that were not captured by our original search, bringing the total to 41 studies. After narrowing the search results using the inclusion and exclusion criteria, 19 preclinical and 22 clinical studies were identified. The timing of decompression in animal models ranges from minutes to 24 hours postinjury, with earlier decompression usually associated with greater neurological improvement. Clinically, the definition of early surgery is generally accepted as that which is undertaken within 24 hours of the initial injury. The evidence available for both preclinical and clinical studies is reviewed here. The majority of these studies showed that either the degree or the duration of compression directly correlated to the degree of recovery. The preclinical literature was reviewed, focusing particularly on (1) histopathological correlation between the injury model and the damage caused to the spinal tissue, (2) animal models that did not show a functional benefit following early decompression, and (3) animal models that showed a functional benefit following early decompression. These studies are summarized in Table 23.1. Three studies examined either the electrophysiological or the histological consequences of spinal cord compression with a fixed duration of time.6–8 The collective results of these early investigations into SCI suggest that direct pressure to the spinal cord, likely resulting in direct damage to the neural cell membranes, combined with hypotension and resultant ischemia, causes a loss of neurological function. Animals that showed recovery following injury demonstrated either a normal microscopic examination of the spinal cord or evidence of central gray necrosis, peripheral demyelination, or laceration. Animals that failed to recover showed more pronounced evidence of damage to the neuroanatomical circuits of the spinal cord at the level of the anterior horn cells or laceration of either the gray or the white matter. Five studies failed to demonstrate a benefit of early decompression following SCI. This generalized conclusion is closely linked to the experimental design of each of these studies. Of those that compared time of compression to outcome,9–11 the maximum time of compression was 2 hours. To elaborate, Croft et al.10 showed that, with a graded pressure and time up to a maximum of 58 g for 20 minutes, the electrophysiological changes observed (somatosensory evoked potentials, SSEP) were completely reversible. The weakness of this investigation was that no statistical analysis was performed. Thienprasit et al.11 subjected a group of cats to a compression model of SCI and then stratified the animals into those that demonstrated electrophysiological recovery within 6 hours and those that did not. Each group would then be stratified to receive decompression or decompression + hypothermia. Of the animals that showed electrophysiological recovery, there was no difference between the control group and the groups that received decompression or decompression + cooling. Of the animals that showed no electrophysiological recovery, there was no difference between the control group and the group that received early decompression; however, the early decompression + cooling did show better behavioral outcomes, suggesting a possible neuroprotective role for hypothermia after SCI. Aki and Toya,9 using a dog model, showed that compression for either 30 minutes or 60 minutes resulted in similar electrophysiological and histological outcomes. The remaining two studies that failed to demonstrate a correlation between the time of compression and outcome attempted to model cauda equina injury12 and studied a novel hydrogel13 with the hypothesis that this agent would act as a scaffold for neural repair following transection . Neither demonstrated an effect of early treatment. The number of animal studies that showed benefit from early decompression far outweighed those that did not. Using a primate model of SCI, Kobrine and others14 showed that the duration of compression correlated to the neurological outcome of these animals and that physical injury to the neuronal membrane could account for a lack of recovery. In a rat model that used five times as many animal subjects, Dolan et al.15 showed that the degree of functional recovery was directly proportional to the duration and force of compression, whereby greater recovery was observed with lower forces and less time of compression. Guha et al.16 further delineated this observation using a rat model and concluded that the major determinant of recovery was the intensity of the compression, and that the time of compression was important only with lighter compressive forces. These results were echoed by a similar study conducted 1 year later.17 Zhang et al.18 expanded on this notion by measuring concentrations of energy-related metabolites in the spinal cord after injury. They concluded that animals with a larger compressive force showed higher concentrations of lactate and inosine in the extracellular compartment of the spinal cord and that these higher concentrations were associated with less neurological recovery. Delamarter et al.19 used a canine model to show that compression of the cauda equina for 6 hours or longer resulted in a lack of significant motor recovery despite decompression. This lack of recovery was associated with central necrosis of the spinal cord. In a set of two experiments using a canine model of SCI, Carlson et al.20 showed Although the neurological outcomes after early decompressive surgery in human clinical studies have been less conclusive than those in animal models, recent work25,26 adds weight to the growing clinical consensus that favors early surgical decompression for patients with an acute traumatic SCI. Studies have focused on the safety and feasibility of early surgery in addition to improvement in neurological function. The clinical evidence, according to the level of evidence of each study, is reviewed here. The studies are summarized in Table 23.2. No level I evidence exists to guide clinicians with regard to the timing of surgical decompression following SCI. Three level II studies were identified.27,28 Vaccaro and others28 studied 62 patients who presented with a spinal injury between C3 and T1. They defined the early surgery group as those who were treated within 72 hours and the late surgery group as those who were treated after 5 days. These authors found no difference between groups with regard to the length of stay in the intensive care unit or inpatient rehabilitation and no difference with regard to the American Spinal Injury Association (ASIA) motor score. In contrast, Cengiz et al.27 studied 27 patients who sustained a traumatic SCI from T8 to L2. They defined early surgery as that occurring within 8 hours of injury and late surgery as that occurring from 3 to 15 days after surgery. There were several differences between the groups at follow-up. The early surgery group showed more improvement on the ASIA Impairment Scale, no in-hospital complications, and a shorter length of stay both in hospital and in the intensive care unit (ICU). The later surgery group had four complications: three cases of lung failure and one case of sepsis. The authors concluded that there are statistical differences between patients treated early and those treated late, with regard to both neurological improvement and overall morbidity. There were no mortalities in either group.
Timing of Surgery for Acute Spinal Cord Injury: From Basic Science to Clinical Application
 Systematic Review Methods
Systematic Review Methods
 Results
Results
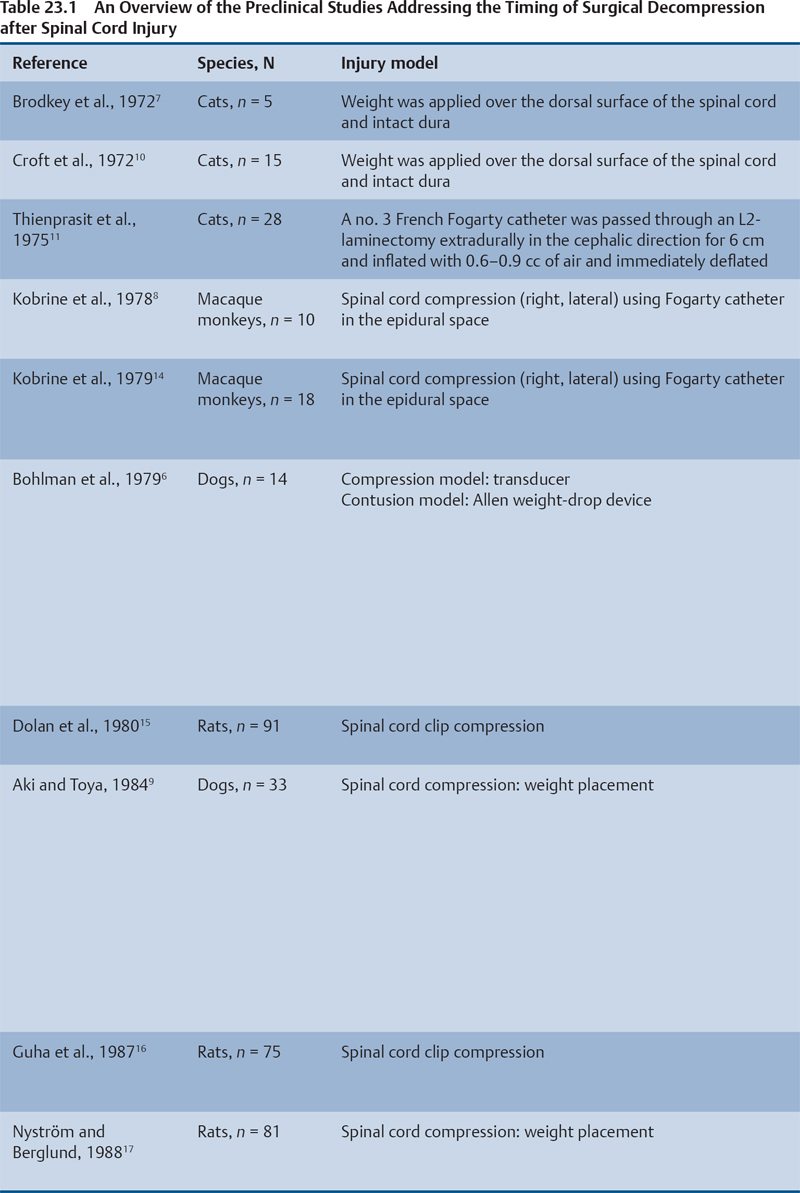
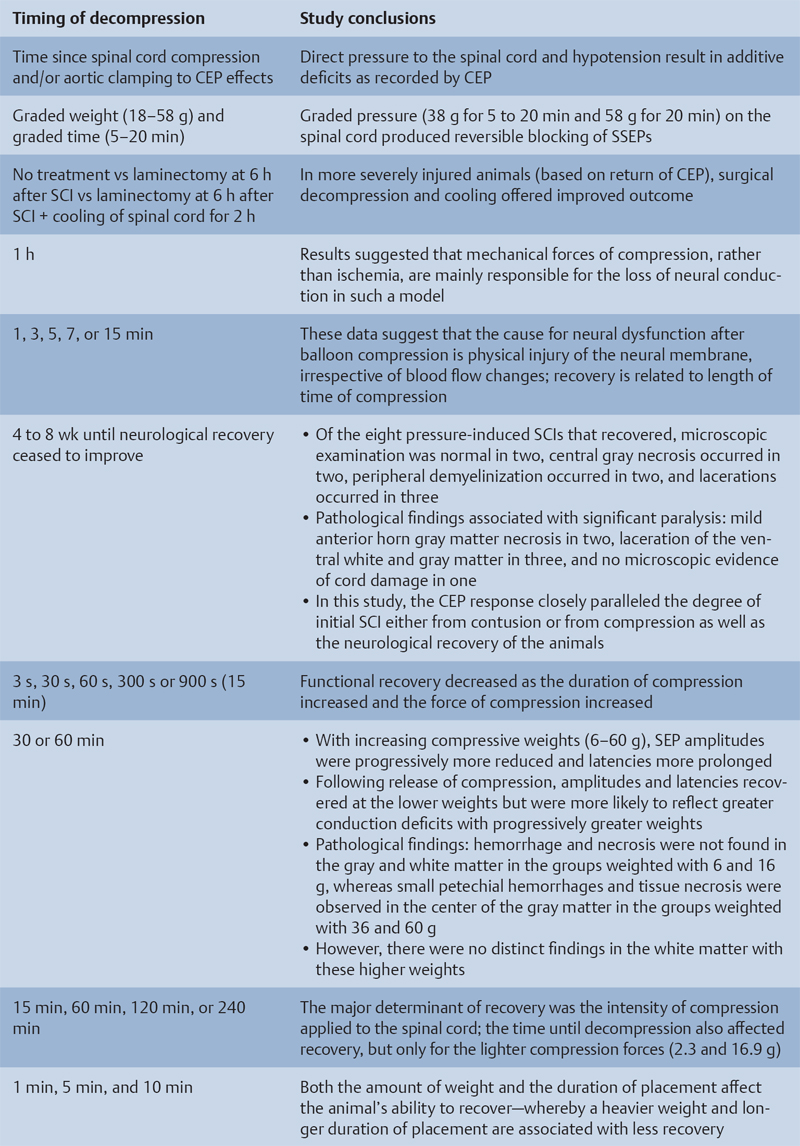
Preclinical Literature
Histopathological Correlation
Animal Models That Showed No Benefit from Early Decompression
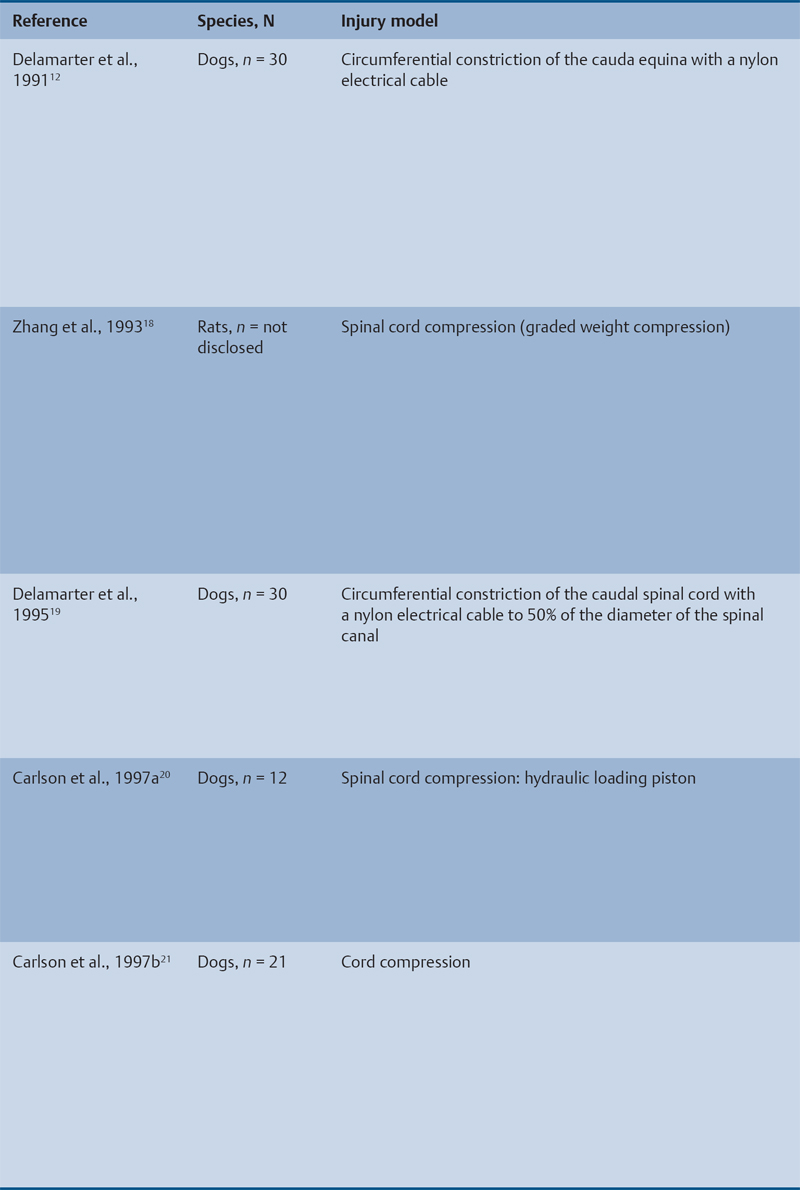
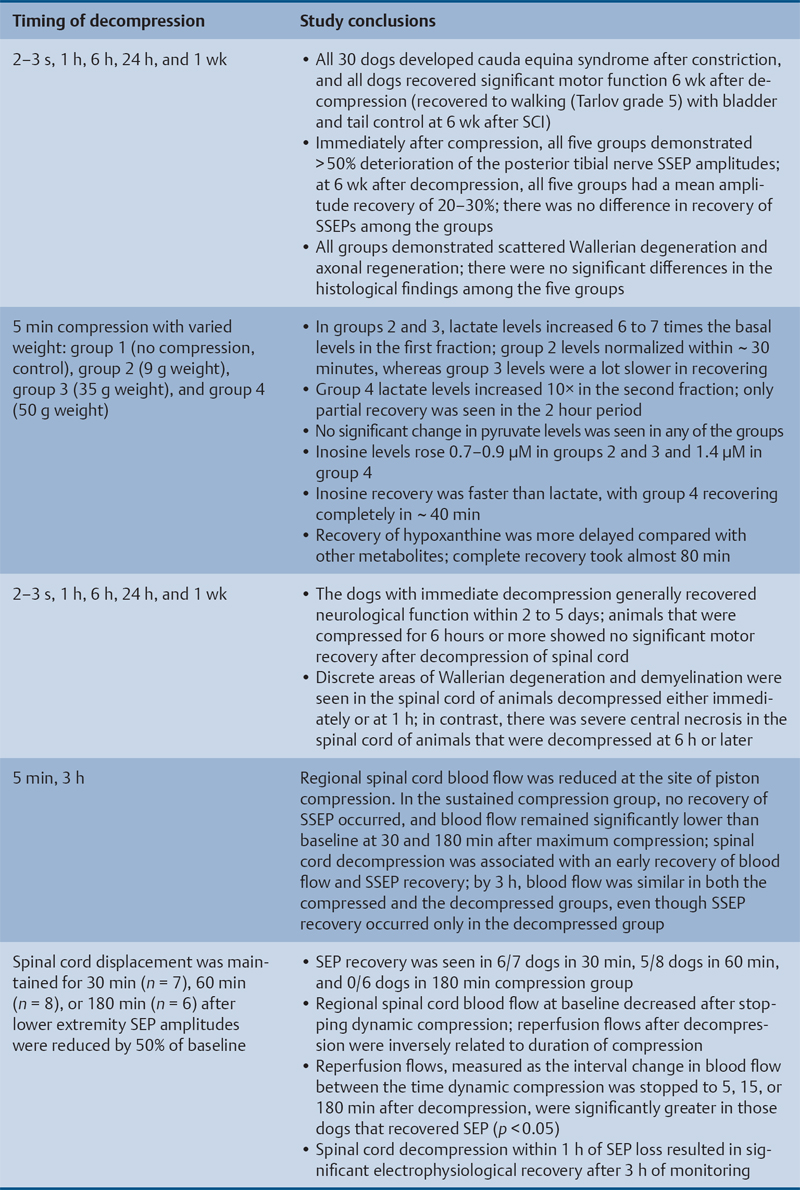
Animal Models That Showed Benefit from Early Decompression
Human Clinical Trials
Level I
Level II