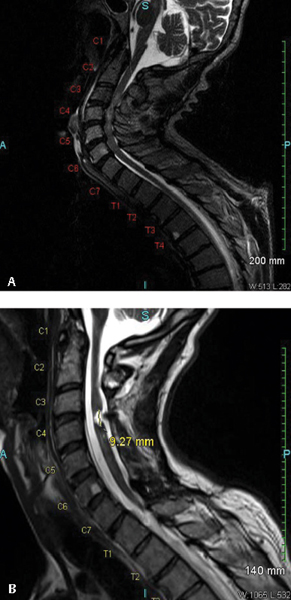
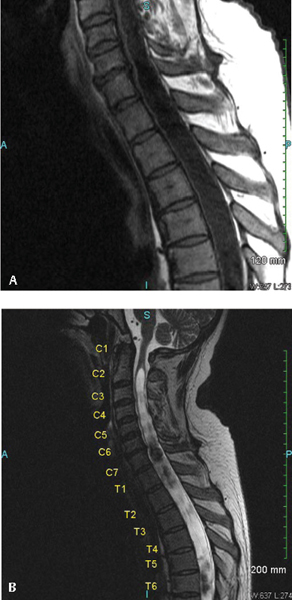
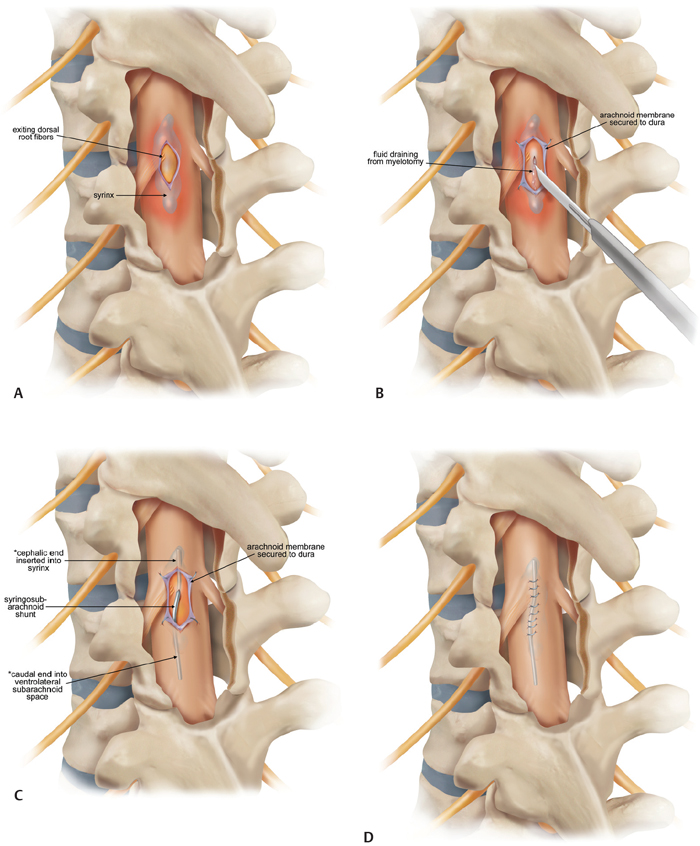
20 Key Points 1. Posttraumatic syringomyelia should be considered in patients experiencing subacute or chronic neurological deterioration after spinal cord injury. 2. Magnetic resonance imaging is the diagnostic modality of choice, with the syrinx most easily observed on sagittal T2-weighted sequences. 3. Comprehensive workup is required to rule out other causes of syringomyelia, including Chiari malformation, congenital tethered cord, and neoplastic disease. 4. Goal of posttraumatic syrinx treatment is disease stabilization, with therapeutic interventions ranging from simple spinal decompression, syrinx shunting, adhesiolysis and expansion duraplasty, to cord detethering. Syringomyelia is characterized by the presence of abnormal fluid-filled cavities in the substance of the spinal cord. Syrinx etiology can be associated with a very diverse and heterogeneous group of diseases, ranging from hydrocephalus, associated structural malformations, infection or inflammation, spinal cord infarction or hemorrhage, to trauma. This chapter focuses on syringomyelia that occurs after spinal cord injury (SCI) and reviews the epidemiology, pathophysiology, and clinical management of posttraumatic syringomyelia (PTS). Radiographic and postmortem detection of syringomyelia occurs in up to 30% of patients with spinal cord trauma, with series variation based on the extent and pattern of injury.1–5 Clinical presentation from such lesions occurs in fewer than 10% of the SCI population, most frequently in men, correlating with the trends in injury incidence.3 In a prospective longitudinal study of 449 SCI patients, Schurch and coworkers observed the development of symptomatic syringomyelia in 4.5% of patients over 6 years. Of note, however, is that PTS can also occur in a delayed fashion at much longer follow-up.4 Vannemreddy and coworkers6 retrospectively reviewed factors predisposing to the development of PTS in a series of 58 symptomatic patients. They found that onset of symptomatic PTS occurred earlier among patients with more advanced age, cervicalor thoracic-level injuries, complete neurological injury at the index level, and fracture-dislocations, especially if patients underwent surgery for deformity correction and stabilization. Advanced age may be associated with acquired canal stenosis, promoting the likelihood of syrinx formation in the traumatized cord.5,6 The association of earlier syrinx development with greater neurological injury and fracture dislocations requiring surgical intervention may reflect the greater force severity applied to the spinal cord and consequent increased inflammatory activation.6–8 Beyond injury to the neural tissue, deformity may contribute to PTS as a result of meningeal injury and adhesion, impaired circulation of cerebrospinal fluid (CSF), and altered vascular supply.6,9,10 Cervical spine injury may promote syrinx development by local tethering at the naturally more mobile site, which is exacerbated in quadriplegics, for whom head and neck movement is the most significant remaining motor function.6,8,11 PTS location is expectedly most frequent at the injured level. Although rostral extension reportedly occurs in more than 96% of cases, isolated caudal extension rarely occurs (4%).1,8 The remarkably low incidence of the latter may result from decreased detection because patients with extension below the level of injury may not develop symptoms to warrant investigation. Longitudinal extent of the syrinx is highly variable, with one of every six cases reportedly spanning more than 10 levels.12 The mechanism by which PTS develops remains speculative, although most theories support a two-step process by which the predisposing pathoanatomy is established at the time of initial injury, and propagation occurs by secondary mechanisms. The primary injury can cause an early cystic lesion by mechanical disruption of cord parenchyma, ischemic damage from arterial or venous obstruction, intraparenchymal hemorrhage with subsequent hematoma liquefaction, and proteolytic enzyme activity or excitatory amino acid activity.13–16 Indeed, the mechanical deformation of the spinal column can also produce a variety of configurational abnormalities that may contribute to syrinx progression. Spinal stenosis, subarachnoid adhesions, and deformity are common among SCI patients who develop PTS, presumably reflecting the severity of the index injury and thereafter contributing to the generation of repeated microtrauma and impacting CSF circulation on an ongoing basis. Posttraumatic syringes are associated with lesions localized to the dorsolateral and central gray matter, and dystrophic cavitations secondary to the ischemic or hemorrhagic insult. Unlike hydromyelia, wherein the cyst walls are lined by ependymal cells of the central canal, the cyst walls of PTS can be irregular in contour, with walls formed by compressed or gliotic tissue, containing microglia and hemosiderin-laden macrophages.12,17,18 Cellular changes include Wallerian degeneration of the associated neurons, with demyelination of the white matter and neuronophagia in the gray matter.12,18 Fibrotic thickening of the leptomeninges occurs, reflecting the underlying process of arachnoiditis observed during surgical management.17 The normal astroglial response to injury is reactive, with release of growth factors that promote neuronal survival19; however, any concomitant disturbance of this delicate healing balance can alternatively promote astrogliosis that generates both physical and biochemical barriers to neural regeneration.20–22 Further consequences of an excessive healing response may be the initiation of an adhesive arachnoiditis at the level of the sustained injury.7,23,24 In this setting, trauma to the arachnoid alone may cause progressive syringomyelia by spinal cord tethering and alteration of CSF circulation, with consequent expansion of intramedullary microcystic lesions into macroscopic syringomyelia lesions. Josephson and coworkers25 describe this theory of CSF pulse pressure against an area of thecal sac constriction dividing the spinal canal into two separate hydrodynamic compartments, with consequent pulsatile activity against an area of high relative local pressure. This uncoupling of the pulse pressure in the CSF and spinal cord leads to higher intramedullary pressure directed centrifugally, with consequent macroscopic cyst formation and subsequent expansion as the proximal component is actively compressed and the distal portion is passively distended. Using a constriction model in Sprague-Dawley rats, edema was detectable by magnetic resonance imaging (MRI) within 3 weeks of injury, and cystic cavitation developed between 8 and 13 weeks postinjury. Similar computational studies by Bilston and coworkers26 identify the peak pulsation pressure to depend on obstruction permeability, facilitating fluid flow into the spinal cord and consequent syrinx expansion. Although this mechanism of PTS development remains dependent on a primary process leading to microcystic cavitation, it underscores the value of surgical intervention to address the healing response as well as any primary bony and neural injury. Patients with PTS most commonly present with pain. Motor deficit and dissociated sensory loss are also common features.4,8,27–29 Pain history is often variable, mild to severe, and intermittent to constant, reflecting injury to the spinothalamic pathway, and pain is consistently exacerbated by maneuvers that increase intracranial pressure and positional changes.8,27,28 The classic dissociated sensory loss (loss of pain and temperature sensation with preservation of light touch and proprioception) reflects intact dorsal column functionality and is more common than a complete sensory loss.8,27,28 Indeed, the selective loss of pain and temperature sensation in conjunction with an ascending sensory level is a very sensitive indicator for detecting progressive PTS.4 Motor symptoms reflect injury to the neighboring lateral corticospinal tract, with an upper motor neuron pattern on clinical examination. Less common symptoms include hyperhidrosis, autonomic dysreflexia, Horner syndrome, and respiratory insufficiency. Bladder hyporeflexia and abnormalities of bowel function occur, normally in conjunction with other symptoms and signs, but have been reported to be the isolated presenting features.18 Further, syrinx extension into the caudal medulla may also lead to cranial nerve involvement, and physical examination should include evaluating for signs of such involvement. The density of bone forming the spinal column makes plain computed tomography (CT) ineffective in establishing the diagnosis of PTS. Enhancement of this technique with contrast myelography provides for detection of associated adhesions, although with poor sensitivity, missing the diagnosis of PTS in up to half of cases. Excellent soft tissue discrimination afforded by MRI provides enhanced sensitivity and is the test of choice to establish the PTS diagnosis, demonstrating either a focal cystic lesion (Fig. 20.1) or a diffuse, longitudinal, expansile lesion (Fig. 20.2).30,31 Additional information about syrinx pathoanatomy is also provided, such as lesion symmetry, loculation, and multiplicity. The presyrinx state, an entity evolving at present in the Chiari I malformation literature, may also be detected following SCI as a T1-hypointense, T2-hyperintense lesion that does not expand the spinal cord and can precede the development of radiographic PTS.30,32–34 The value of cine MRI remains investigational, although the potential to identify sites of obstructed CSF flow and to observe normalization following intervention may be valuable in the future.35 Fig. 20.1 Magnetic resonance imaging (MRI) study of a 50-year-old male patient who developed posttraumatic syringomyelia as a complication of an unstable C3-4 complex injury. (A) The preoperative T2-weighted MRI scan demonstrates the traumatic and degenerative changes, with (B) the postoperative T2-weighted scan showing a small posttraumatic cyst at the site of previous cord contusion. Fig. 20.2 Magnetic resonance imaging (MRI) study of a 51-year-old male patient who developed posttraumatic syringomyelia as a complication of a T9-level injury. Symptoms include new onset of neck pain and stiffness in a previously T9-paraplegic patient. The expansile cystic lesion spans from C2 to T8 and is seen on both (A) T1-weighted and (B) T2-weighted MRI studies, with the syrinx contents having similar signal intensity to CSF. The need to intervene among patients with PTS is based on a consistent progression of neurological disability, with few reports of spontaneous resolution,36–38 which presumably occurred consequent to spinal cord fissuring, with consequent syrinx decompression into the subarachnoid space. Medical management has a role only in the context of symptomatic treatment for patients with mild disability or who are not surgical candidates by choice or condition. Pharmacological options include antispasticity agents, tricyclic antidepressants and anticonvulsants targeting neuropathic pain, anticholinergic medications to treat excessive secretory activity, and narcotic analgesics for treatment of refractory pain. Surgical indications include those with asymptomatic radiographic syrinx progression, new onset of symptoms following SCI with radiographic evidence of syrinx, or substantial neurological deterioration, pain, or autonomic dysreflexia in patients with known PTS.4,8,28,39 The stated goal of surgical treatment for PTS should be disease stabilization, although variable reports of neurological recovery of neurological deficit have been described, with satisfactory clinical outcome reported in between 80 and 90% of patients.4 Decreased pain and improved motor function are the most common improvements,4 with recovery of disordered sensation and decrease in hyperhidrosis being less predictable.27,29 Effect on limb spasticity is variable and the least improvement is noted in the clinical sign of deep tendon reflexes.4,40 Choices for surgical intervention are variable and highly dependent on the presenting pathoanatomy. Strategies that are commonly employed and that are technically accessible to most neurosurgeons include simple spinal decompression, percutaneous syrinx drainage, syrinx shunting, adhesiolysis and expansion duraplasty, and cord detethering. Limited symptomatic improvement in slightly more than half of patients has been reported for spinal decompression alone in small case series,41 although this may represent a more limited operation that could be reasonably offered in the setting of substantial deformity or severe spinal stenosis. Although reduction of spinal column deformity has been reported,13 with the conferred benefits of potentially treating local obstruction to CSF circulation, using a method that is entirely extradural must be balanced against the surgical trauma, particularly in cases requiring thoracotomy or thoracoabdominal exposures. Simple syrinx fluid drainage without any mechanism for continued evacuation has been associated with only short-lived clinical effect and has been largely abandoned in favor of cyst fluid diversion strategies with more sustained results. Sudheendra and coworkers42 review the literature surrounding this tactic and illustrate the case of a single patient who responded with both clinical and radiographic improvement to fluoroscopic syrinx aspiration, although repeated interventions were required three times over the span of 3 years. Nevertheless, such a technique may represent an adjunct to surgery, temporizing intervention among patients who are not optimized for surgery or improving operative access to the subarachnoid space by collapsing the size of the spinal cord. Shunting of a PTS into the subarachnoid space (syringosubarachnoid) or into a low-pressure cavity, such as the pleural (syringopleural) or peritoneal (syringoperitoneal) regions, may provide effective and sustained diversion of cyst fluid to limit syrinx growth and afford neurological recovery. Figure 20.3 illustrates steps involved in placement of a syringosubarachnoid shunt. Briefly, this involves laminectomy to provide access to the spinal canal, followed by a myelotomy over the thinnest rim of neural tissue surrounding the caudal syrinx, with preference given to the posterior median sulcus or dorsal root entry zone. The shunt is generally introduced in a caudal to cranial direction to minimize the risk of iatrogenic neurological injury. To minimize the possibility of shunt migration, a suture can be placed to secure the shunt to the pia or dura, or T-shaped shunts can be used. Syringosubarachnoid shunt failure can be associated with dense subarachnoid scarring that can lead to obstruction and reaccumulation of the cyst. Placement of the distal end of the syringosubarachnoid shunt in an area free of arachnoid adhesions may decrease the incidence of shunt failure.8,43 Batzdorf and coworkers43 critically appraised the success of the various shunting techniques, finding that half of the patients undergoing such procedures experienced some complication of the procedure at a median of 9 years follow-up. Among those for whom the proximal shunt was removed because of obstruction, ingrowth of glial tissue was found to be the putative material. No difference in the incidence of complication was observed by the different shunting methods, a result previously reported by Umbach and Heilporn.44 Over all, clinical improvement is reported in 12 to 89% of patients following shunt insertion, although these series report up to one third of patients exhibiting postoperative deterioration.8,39,45 Various case series are summarized in Table 20.1, and the significant variability in results likely reflects the heterogeneity of the regional trauma experience with different severity, pattern of spinal column injury, and incidence of subarachnoid scarring. Fig. 20.3 (A) Technique for placement of syringosubarachnoid shunt. A laminectomy is performed to allow access to the dura, which is opened in the midline. (B) The pia is opened next, and pial sutures are placed and a midline myelotomy is performed to allow access to the syrinx. (C) The shunt is then inserted in a caudal to cranial direction into the syrinx. The distal end is placed in the adjacent subarachnoid space. (D) Following satisfactory placement and decompression of the syrinx, the dura is closed in a watertight fashion. For a syringopleural or syringoperitoneal shunt (not shown), the distal end is subcutaneously tunneled and placed in the pleural space or peritoneum, respectively.
Posttraumatic Syringomyelia: Pathophysiology and Management
 Epidemiology
Epidemiology
 Pathophysiology
Pathophysiology
 Clinical Presentation
Clinical Presentation
 Diagnosis
Diagnosis
 Management
Management
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Posttraumatic Syringomyelia: Pathophysiology and Management
