24 THORACIC TRAUMA
EPIDEMIOLOGY OF THORACIC INJURIES
A commonly cited statistic suggests that thoracic injuries are responsible for approximately 20% percent of all traumarelated deaths.1 This citation, however, is not only dated but relied heavily on inference and extrapolation and not use of rigorous scientific methods for data collection. But, as the authors acknowledged, “Few statistics are available in the United States on chest trauma itself’.”1 Today, published statistics on thoracic trauma remain sparse. For the 10-year period from 1996-2005, the trauma registry at the R Adams Cowley Shock Trauma Center (STC) in Baltimore, Maryland, indicates that approximately 5750 patients per annum arrived in the trauma resuscitation unit.2 On average, 28% of admissions were classified as having an injury to the thorax. Of those patients admitted with a thoracic injury, 54% may be classified as a serious thoracic injury. With the use of the Abbreviated Injury Scale,3 “serious thoracic injury” may be defined as a score of >2 (on a scale of 1 to 6, where 1 is considered minor and 6 virtually nonsurvivable). Patients who die and have a serious thoracic injury (as defined above), although potentially other serious injuries may be present, make up 56% of all deaths of all admissions to the STC.2
The epidemic of societal violence has changed the patterns of chest injuries that dominated previous decades. A significant number of violent interactions involving shootings or stabbings result in penetrating chest trauma.4 Likewise, growth in the elderly segment of the population has an impact on the changing pattern of thoracic injury. Today, patients older than 65 years of age seen at the STC have a 30% chance of having thoracic trauma (mild, moderate, or severe).2
THORACIC ASSESSMENT IN TRAUMA
The assessment of patients with thoracic injury is based, as is any type of trauma examination, on a series of diagnostic clues obtained from directed data collection. Initially the data are used to form a diagnostic set known as the index of suspicion. In other words, given the specific details of the incident and the initial, rapid assessment, a list of injuries most likely to be present is identified.5
THORACIC ANATOMY
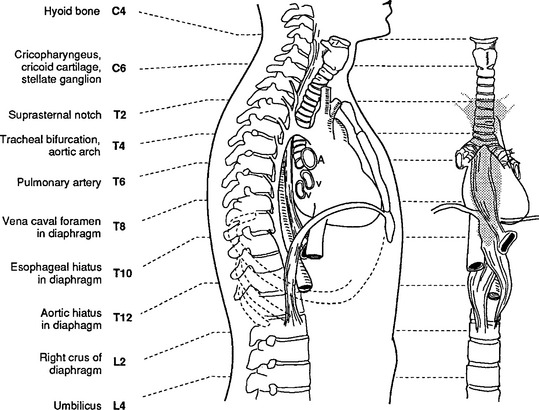
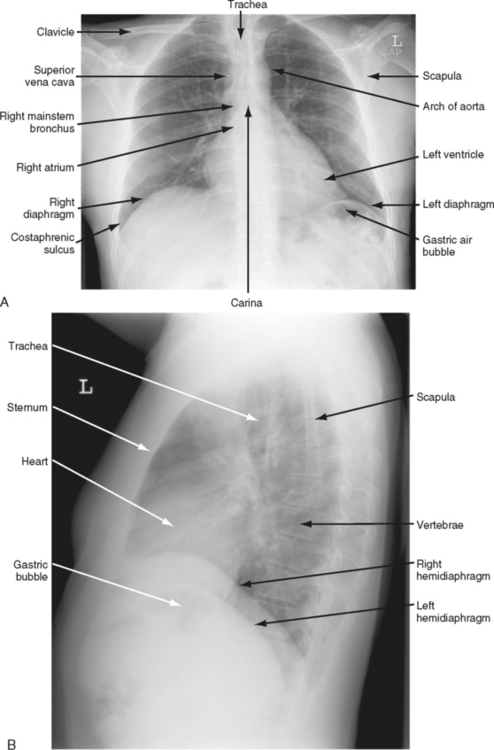
Correlation of underlying anatomy and surface landmarks is imperative in the trauma examination. The examiner must be able to identify key structures in the true thorax, the cervicothoracic inlet, and the boundaries of the thoracoabdominal cavity. Key structures include the trachea, carotid arteries, carina, lung fields, diaphragm, cardiac borders, aorta subclavian arteries, and pulmonary artery (Table 24-1). External landmarks and knowledge of the relationship with internal structures assist in identifying injuries (Figures 24-1 through 24-5).
TABLE 24-1 Thoracic Surface Anatomy
| Structure | Landmarks |
|---|---|
| Aorta | |
| Root | Angle of Louis, midsternal line |
| Arch | First rib, sternal border |
| Pulmonary artery | Within and below aortic arch |
| Subclavian artery | First rib, clavicle |
| Cardiac borders | |
| Apex | Fifth left ICS, midclavicular line |
| Base | Second left ICS, substernal |
| Carina | Angle of Louis |
| Diaphragm | Right dome superior to left |
| Full inspiration | Tenth-eleventh rib posteriorly, sixth-eighth rib anteriorly |
| Full expiration | Tenth thoracic vertebra posteriorly, fourth-fifth rib anteriorly |
ICS, Intercostal space.
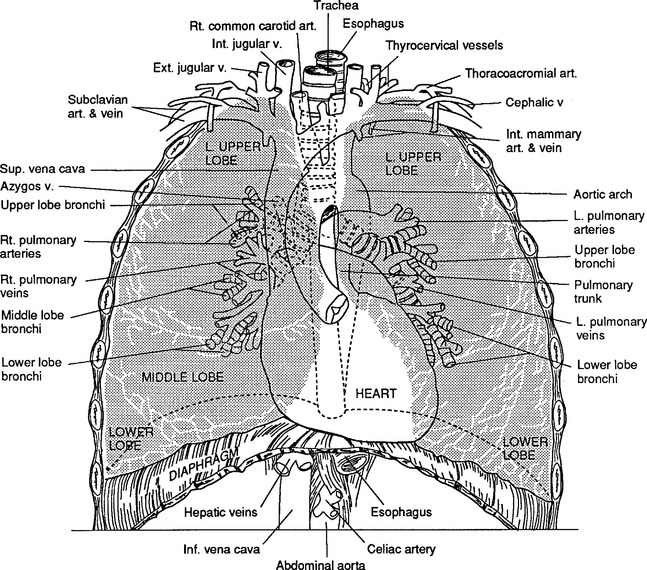
FIGURE 24-1 Anatomy of the thorax and its contents.
(From Shires GT: Care of the trauma patient, New York, 1985, McGraw-Hill.)
PHYSICAL EXAMINATION
Primary Survey
Ensuring adequate circulation is the next priority. Although blood pressure (BP) and pulse are noted, neither is a definitive sign of shock. The heart rate may be elevated in the trauma patient as a result of pain and anxiety, even in the absence of significant injury. Conversely, patients prescribed β-blockers may have a relatively slower heart rate than expected. Low BP is a late marker of shock6 and not recommended as a sole indicator of poor tissue perfusion.4,6 This is particularly true in young patients who have the ability to vasoconstrict and maintain a relatively normal BP despite significant intravascular volume loss. Warm extremities, brisk capillary refill time, normal mentation, and adequate urine output indicate an intact cardiovascular system providing adequate tissue perfusion. The neck veins are assessed for distention. Although jugular venous distention (JVD) may accompany injuries such as tension pneumothorax and cardiac tamponade, these conditions may be present even in the absence of JVD, which may not be evident because of hypovolemia.
During the initial survey, the presence or suspected presence of a pneumothorax or hemothorax is treated with a thoracostomy tube. A tension pneumothorax, considered a serious and often life-threatening form of pneumothorax, requires immediate decompression, which may be initially achieved by inserting a large-bore needle (e.g., 14-gauge) into the second intercostal space over the rib margin in the midclavicular line of the affected side. The tension pneumothorax is thus converted to a pneumothorax, and treatment with a thoracostomy tube may be pursued.
Finally, the patient’s axillas are assessed and the patient is turned and the back inspected, and the number and location of penetrating wounds or blunt injuries are noted. To turn the patient who had sustained blunt trauma requires “log-rolling,” although this technique is not always an imperative in penetrating injuries. Clinical judgment is the best guide as to whether log-rolling is indicated in the latter group.7
PHYSIOLOGIC APPROACH
Clinical monitoring of BP, cardiac rate and rhythm, respirations, urine output, and ABGs is considered a standard part of patient management after thoracic injury. However, it is recognized that BP is frequently unaffected until a 30% loss in blood volume has been sustained.4 Although invasive monitoring of central venous pressure may be helpful in evaluating intravascular volume, it is not routinely initiated during early fluid replacement and management of significant thoracic injuries. The time required to insert a central venous catheter cannot be afforded during initial moments of resuscitation; therefore, large-bore (14-gauge) peripheral intravenous catheters are preferable for volume repletion. If the patient has limited intravenous access, a central venous line can be placed.
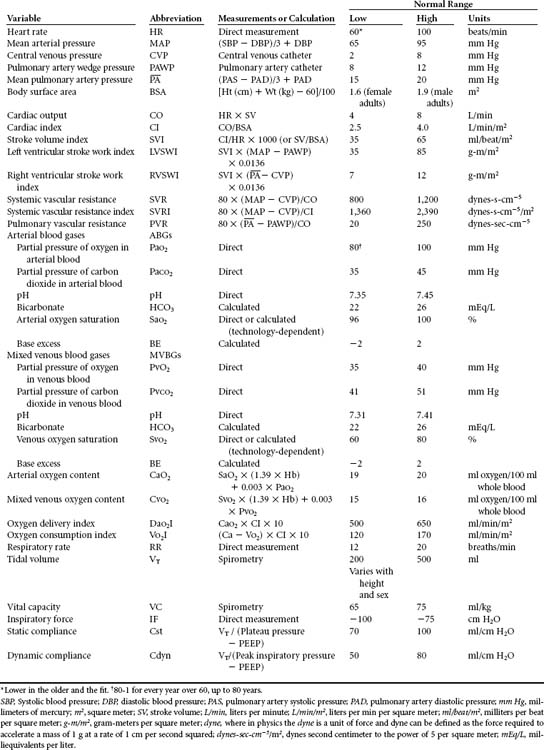
Arterial and pulmonary artery catheters provide information useful for assessing patients with extensive injuries and related complications. These hemodynamic parameters can also aid in determining the effect of therapies such as inotropes, fluids, and positive end-expiratory pressure (PEEP). When indicated, mixed venous oxygen tension and saturation monitoring may be helpful tools in determining circulatory sufficiency and global tissue oxygen consumption. In particular, geriatric patients, including the apparently clinically stable group, with multiple fractures, head injury, acidosis, initial systolic BP <150 mm Hg, or who were pedestrians struck by a motor vehicle may benefit from early invasive monitoring coupled with judicious use of vasoactive drugs.8,9 Table 24-2 is a summary of physiologic parameters that may be obtained to monitor the patient with thoracic trauma.
NONINVASIVE MONITORING
The oxygenation status of the patient can be assessed by using noninvasive monitoring with pulse oximeters and transcutaneous, conjunctival, or tissue oxygen tension monitors. Oximetry is the determination of the oxygen-hemoglobin saturation of the blood and may be measured with an appropriate sensor that detects the pulsatile blood flow through some translucent part of the body (e.g., fingers, ear lobes, nasal septum, forehead). Pulse oximetry (SpO2) is well accepted as a convenient, portable, noninvasive, and cost-effective indicator of arterial oxygen saturation (SaO2). Pulse oximetry estimates the fractional hemoglobin saturation by determining the maximal light absorbance of the different hemoglobin species. Practical application of this technology aids both the detection of hypoxemia (SaO2 <90%) and hyperoxemia (SaO2 >98% in the presence of supplemental oxygen), allowing for appropriate titration of inspired oxygen concentration and other mechanical ventilator settings. SpO2 measures are up to 4% higher than SaO2 when at 90%, assuming that the only hemoglobin species present are reduced hemoglobin and oxyhemoglobin. The accuracy of SpO2 measures may be reduced in the presence of movement, significant amounts of carboxyhemoglobin or methemoglobin, dark skin pigmentation, hypothermia, and hypovolemia, although advancements in technology continue to negate the impact of these variables. Anemia, once considered to affect accuracy, may have only a minor impact on the precision of measurements with the pulse oximeter.10 Because pulse oximetry depends on pulse transmission, intense peripheral vasoconstriction may also be associated with lost or inaccurate readings. Generally, when the SaO2 is greater than 90%, the accuracy of the SpO2 increases substantially. An SpO2 of less than 92% serves as a trigger to consider additional tests to confirm the oxygenation status of the patient.10,11
Noninvasive assessment of carbon dioxide (CO2) is complex, especially in critically ill patients with significant ventilation-perfusion (V/Q) abnormalities, increased physiologic dead space, and hemodynamic instability. When these confounding factors are stable, changes in end-tidal CO2 (ETCO2) can be assumed to reflect changes in alveolar ventilation and arterial CO2 tension (PaCO2). ETCO2 monitoring by mass spectrometry or an infrared analyzer correlates reasonably well with PaCO2 and is used in a variety of environments, including prehospital, emergency department (ED), and operating, recovery, and critical care areas.12 In relatively healthy patients, the relationship between PaCO2 and ETCO2 is close (less than 5 mm Hg difference), with the exhaled CO2 (ETCO2) commonly lower than the PaCO2. For example, a PaCO2 of 40 mm Hg might correlate with an ETCO2 of 36 mm Hg.12 As with other forms of monitoring, analysis of a trend over time rather than an individual value is most useful because there may be considerable breath-to-breath variability. Currently, there is some interest in the use of ETCO2 to identify patients requiring more aggressive resuscitation during emergency trauma surgery13 and as a predictor of death in patients undergoing emergency trauma surgery.14
Sublingual capnometry may be destined to play a role as a noninvasive indicator of the depth of shock and adequacy of resuscitation. Sublingual capnometers consist of three components: a disposable CO2 sensor (placed under the tongue, equilibrates with the corresponding CO2 level in the superficial mucosa), a fiberoptic cable, and, a blood gas analyzer. Within 5 minutes of connecting the components, a sublingual CO2 measurement is attained. Such values may be useful as end points of resuscitation.15
INTEGRATING OTHER DIAGNOSTIC DATA
Thoracic evaluation commonly includes a CXR to visualize any significant bony, vascular, or pulmonary injuries (Figure 24-6, A and B). The appropriate angle of the film (supine, upright, or lateral) is used for best exposure of specific structures yet must consider any restrictions in positioning the patient. Often, as a result of hemodynamic instability or other suspected injuries (e.g., spinal column fracture), the CXR is a portable, supine, anteroposterior (AP) view. For penetrating injuries, a radiopaque marker is placed at suspected entrance and exit sites.16
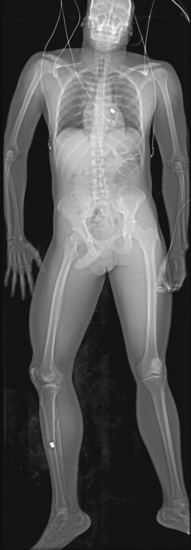
Digital total-body radiographic scanning (StatScan) may aid in the diagnosis of thoracic injury. StatScan is a comprehensive diagnostic total body x-ray examination that can be completed in less than 5 minutes and at a greatly reduced overall radiation dose to medical staff and patients. Initial images are available in less than 10 seconds. The quality of the x-ray film is reasonable although generally limited to one view (AP). StatScan plays a role in quickly and simultaneously identifying abnormalities of the thorax and the rest of the body17 (Figure 24-7).
Overall, the use of plain radiographs is decreasing in the acute trauma setting in part because of the increased use of CT scanning. The CT scan is more sensitive in detecting hemothorax, pneumothorax, and pulmonary contusion than is the CXR.18 Evaluation of underlying lung, cardiac, and mediastinal structures is also enhanced by the use of CT scans. However, a simple and rapid assessment of blunt trauma patients with CXR and abdominal ultrasonography may alert the clinician to significant injury.19 Aortography remains the standard for evaluating aortic branch vessel injury,20,21 although a contrast-enhanced CT may have a sensitivity and negative predictive value equivalent to that of aortography for assessment of just the aorta.22,23 An abnormality of a branching vessel would be an indication to proceed with aortography. Assessment of penetrating trauma may also undergo a decline in use of angiography and other invasive tests as the role of contrast-enhanced CT is expanded.24 As CT technology advances, the potential applications are increasing.25
Three-dimensional (3-D) images of the thorax can be obtained after multidetector row CT scanning. Certain structures may be deleted on the radiograph to allow better visualization of other structures. 3-D imaging plays a role in clarifying complex vascular and nonvascular anatomy. Further technical developments will help solidify the role of two- and three-dimensional reconstructions in the management of thoracic trauma26 (Figure 24-8).
Ultrasonography of the thorax is a relatively new diagnostic tool. Although the surgeon-performed focused assessment by sonography for trauma (FAST) examination has become a standard part of the abdominal assessment for hemorrhage, ultrasonography of the thorax awaits mainstream acceptance and use. The exception, however, is sonography for rapid diagnosis of traumatic pericardial fluid collections.27 Recently, use of sonography for the diagnosis of pneumothorax has gained more interest.28,29 Although posttraumatic occult pneumothoraces may be more likely to be identified by ultrasonography than by a plain CXR,30 the accuracy of ultrasonography continues to be limited by the expertise of the user.
The use of MRI in the acutely ill patient with thoracic trauma is limited by the incompatibility of many metallic implants and life support devices with the magnetic field. Coupled with limited visualization and the potential for rapid hemodynamic deterioration while the patient is in the scanner, MRI is typically not a desirable diagnostic strategy for this group of patients. The use of MRI may be considered an adjunct diagnostic tool in appropriately selected patients, although it will probably have an increasing role as MRI-compatible physiologic support and monitoring devices become increasingly available.25
REASSESSMENT AND EARLY THORACOTOMY
Repeated thoracic assessment is the key to determining missed or progressive injuries. Injury to the thorax requiring intervention may be broadly split into three categories: those requiring observation, tube thoracostomy (chest tube), and formal thoracotomy. The majority (85%) of thoracic trauma cases requiring intervention are managed with tube thoracostomy (chest tube), pain control, pulmonary toilet, and observation.31
1. Penetrating thoracic trauma with a systolic BP <40 mm Hg or prehospital signs of life
2. Penetrating nonthoracic trauma, noncranial trauma with a systolic BP <40 mm Hg and signs of life in the resuscitation area
3. Cardiac arrest after blunt chest or abdominal trauma after arrival in the ED with an obtainable BP16
Successful outcomes after ED thoracotomy are often related to reversible conditions such as cardiac tamponade, controllable intrathoracic bleeding, elimination of massive air embolism, or bronchopleural fistula. Thoracotomy also permits open cardiac massage and access to the descending aorta to facilitate temporary clamping to redistribute limited blood flow to the myocardium and brain while stifling any subdiaphragmatic bleeding.32 Historically, patients were more likely to survive an ED thoracotomy if they had sustained a penetrating injury (5% to 8% survival) than a blunt trauma (<2%) or were in extremis after extrathoracic injuries (<1%).33 Recently, a small European study suggested that patients with blunt trunk trauma and cardiac arrest after hemorrhagic shock may benefit from ED thoracotomy and open massage with a similar probability of survival as shown for patients with penetrating injury. However, the success of the intervention was contingent on early thoracotomy after closed chest resuscitation of less than 20 minutes by trained professionals. On average, the survivors of open chest cardiac massage had undergone 13 minutes of closed chest cardiopulmonary resuscitation before the thoracotomy.34
Thoracoscopy may be useful in limited emergency situations. This procedure may be used on stable patients with penetrating chest injuries, for diagnostic evaluation, or to control bleeding and remove blood clots.35
INJURIES
AIRWAY OBSTRUCTION
The Patient With a Natural Airway
When positioning does not relieve the obstruction, endotracheal intubation is the management technique of choice for most types of trauma. Alternatives to conventional intubation include use of the Laryngeal Mask Airway (LMA) (Laryngeal Mask Company) or the esophageal Combitube (Sheridian Catheter, Argyle, NY). These devices are blindly inserted into the pharynx and on inflation of their seal allow for ventilation of the lungs. The Combitube is relatively easy to insert, even after only brief training. The LMA has a less well defined role in the trauma population and is generally not tolerated if the gag reflex is present.36
Although oral-tracheal intubation constitutes the preferred airway management, there are several caveats. If the patient is unstable but breathing and the urgency of airway management does not allow preliminary cervical spine clearance, blind nasotracheal intubation may be attempted. Conversely, an oral-tracheal route is used if the patient is apneic and the cervical spine is immobilized manually in a neutral position. Last, fiberoptic laryngoscopy and bronchoscopy may be useful to facilitate difficult intubations in stable patients, particularly in those individuals with maxillofacial or cervical spine trauma and in patients with short necks.37 Scopes may be less practical in the emergency or urgent situation.
If the patient cannot be intubated successfully, emergency cricothyroidotomy is recommended. In the event a surgical airway is required, a cricothyroidotomy is preferred over a tracheostomy. Comparatively, a cricothyroidotomy is less bloody, quicker, and considered easier to perform.37 There are two cricothyroidotomy procedures currently in use. The first is a surgical technique in which a transverse incision is made through the skin and the cricothyroid membrane, located below the thyroid prominence of the neck. Usually, a No. 6 endotracheal tube is inserted into the exposed airway (Figure 24-9). A second approach, needle cricothyroidotomy or percutaneous transtracheal ventilation, is initiated by insertion of a 14-gauge needle into the trachea at the cricothyroid membrane below the level of obstruction. Pressurized oxygen is insufflated intermittently through the needle into the trachea. The limitation of the needle cricothyroidotomy is that ventilation is usually impaired despite sufficient oxygenation. The choice of method depends on the injury, available equipment, and capability of the resuscitating health professional.
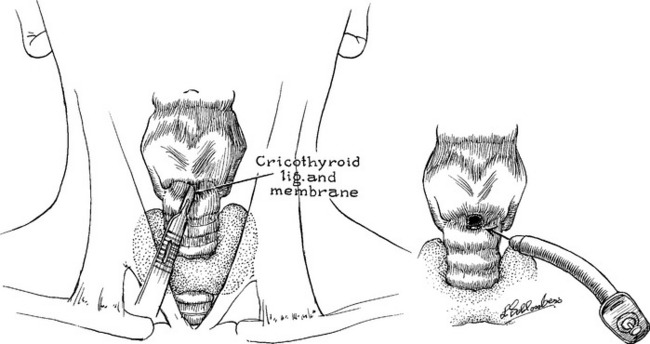
FIGURE 24-9 Cricothyroidotomy technique.
(From Zuidema GD, Rutherford RB, Ballinger WF: Management of trauma, 4th ed, Philadelphia, 1985, W. B. Saunders.)
The choice of appropriate airway requires consideration not only of available equipment and personnel but also factors specific to the patient, the injury, and short- and long-term management plans. For example, early intubation may be indicated not only for airway management but also for intraoperative or critical care management of patients with thoracic injuries. Table 24-3 summarizes the advantages of different artificial airways, their restrictions, and their potential complications.
TABLE 24-3 Airway Adjuncts in Trauma
| Oropharyngeal Airway | |
| Indications | Unconscious patients without gag reflex; short-term use |
| Advantages | Holds tongue away from posterior pharynx |
| Restrictions | Oropharyngeal injuries |
| Complications | Intraoral injury; induction of vomiting and aspiration; increased obstruction if positioned incorrectly by pushing the tongue back into pharynx |
| Nasopharyngeal Airway | |
| Indications | Semicomatose or arousable patients with decreased control of upper airway; prevention of tissue trauma during frequent nasotracheal suctioning |
| Advantages | Better tolerated in awake patients than oral airway; easily secured |
| Restrictions | Maxillofacial trauma such as nasal, nasoethmoid fractures |
| Complications | Nasopharyngeal injury; nasal bleeding |
| Esophageal Obturator Airway, Combitube, Pharynotracheal Lumen Airway, Laryngeal Mask Airway | |
| Indications | When unable to successfully place endotracheal tube |
| Advantages | Can be positioned quickly without direct visualization, with minimal manipulation of cervical spine |
| Restrictions | Cannot be used in awake or semiconscious patients |
| Complications | Induction of vomiting and aspiration; esophageal tears; postpharyngeal bleeding; unrecognized incorrect placement |
| Endotracheal Tube | |
| Indications | Preferred method of airway control |
| Advantages | Stable airway; provides protection from aspiration; permits mechanical ventilation to be used; decreases gastric distention associated with bag-mask ventilation |
| Restrictions | Used with caution in presence of laryngotracheal injuries (glottis, subglottis, and upper trachea) |
| Complications | Esophageal intubation leading to hypoxia; right mainstem bronchus intubation; induction of vomiting and aspi- ration; vocal cord injury; pharyngeal injury; tracheal lacerations; conversion of cervical spine injury without neurologic deficit to injury with deficit; dislodged tube |
| Cricothyroidotomy | |
| Indications | When intubation does not relieve obstruction or trachea cannot be intubated |
| Advantages | More rapid, greater ease of accessibility, and lower incidence of bleeding than tracheostomy |
| Restrictions | Children younger than 12 years old; laryngeal injury or inflammation |
| Complications | Subglottic stenosis; vocal cord injury; aspiration; hemorrhage; tracheal or esophageal laceration; mediastinal emphysema; dislodged tube |
| Standard Tracheostomy | |
| Indications | When intubution does not relieve obstruction or in significant laryngeal or tracheal trauma; used for prolonged ventilatory support |
| Advantages | Bypasses upper airway and glottis; stable airway with low resistance to air flow; easily suctioned |
| Restrictions | Limited use as an emergency procedure because of time requirements and potential for bleeding |
| Complications | Early or delayed hemorrhage; aspiration; mediastinal emphysema with or without pneumothorax; tracheoesophageal fistula; tracheal stenosis; tracheomalacia; tracheoarterial fistula; dislodged tube |
The Patient With an Artificial Airway
In trauma patients with an artificial airway in place (routinely an endotracheal or tracheostomy tube), obstructions or partial obstructions may occur, usually in an insidious and subtle fashion. Obstruction may be caused by thick or dried secretions or blood clots within the lumen of the airway or malposition of the airway. Assessment of airway patency and effectiveness requires continuous evaluation. Signs of airway obstruction include increasing level of agitation, rising airway pressures during mechanical ventilation (peak inspiratory pressure, not plateau pressure), difficulty in advancement of a suction catheter, and decreased SpO2 or increased ETCO2. Frequent evacuation of bloody clots or mucus plugs may precede the occlusion of the airway. Humidification is a front-line measure to prevent secretions from drying in the artificial airway and causing obstruction. Inhalation of aerosolized agents aimed at loosening secretions (e.g., a mucolytic agent such as Mucomyst [acetylcysteine solution]) may also be prescribed. Ensuring that any artificial airway is well secured and supported during repositioning of the patient helps maintain appropriate placement of the device.
The optimal timing of converting an artificial airway from endotracheal to tracheostomy is controversial. Historically, a patient requiring an artificial airway for less than 10 days was managed with an endotracheal tube, and if artificial ventilation was still required after 21 days, a tracheostomy was recommended.38 More recently, advantages to earlier tracheostomy have been suggested.39,40 Advantages include shorter duration of ventilation, shorter intensive care unit (ICU) stay, less damage to the mouth and larynx, and facilitation of communication.
General nursing management begins by documentation of airway type and size, patient tolerance, any complications, duration of intubation, and date of tracheostomy. The patient-specific plan of care is based on such factors as a history of any airway problems, difficulty in intubation, and patient behavior, such as attempts at self-extubation or bronchospasm during suctioning. An identical spare tracheostomy tube, and second with an internal diameter 1 mm smaller than the original, and a manual resuscitator bag with face mask should be located at the bedside for rapid management of obstruction or lost airway. Emergency intubation kits that contain all the necessary equipment for rapid intubation and airway management should be strategically located on the unit. Airway hygiene is implemented on the basis of assessment findings. Tracheostomy care is patient specific depending on the newness of the stoma, the type of secretions or peritracheal drainage, and signs of infection. Commonly, the tracheostomy site is cleaned with saline solution and a gauze dressing is placed around the site at least once a shift and as required. A record is maintained of all tube changes, tube size, and any difficulties encountered in tube placement. The need for long-term airway management is apparent in the intermediate care setting, if not before. An alternative mode of communication (e.g., writing, use of a letter or picture board) must be established for the patient unable to verbally express himself or herself because of placement of an artificial airway. A speech-language pathology consultation may be helpful in determining an alternative means of communication. Insertion of a fenestrated or “talking” tracheostomy will help the patient regain the ability to verbally communicate. As soon as the patient and family indicate readiness, a teaching plan is begun that covers long-term and home management of secretions and tracheostomy care. Excellent reviews of nursing care of the patient with an artificial airway are available.41,42
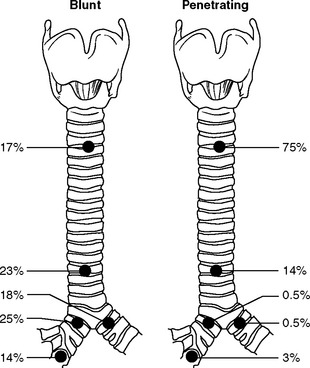
TRACHEOBRONCHIAL TRAUMA
Description
The tracheobronchial tree may be injured by either blunt or penetrating trauma at any level. Most commonly, injury involves the mainstem bronchi within close proximity to the carina in blunt trauma and the cervical trachea in penetrating trauma (Figure 24-10). Injuries may be complete or incomplete, and total separation of the tracheobronchial tree can occur. However, continuity of the airway may be maintained by the fascia surrounding the trachea and bronchi. Lower airway injuries are of interest to nurses in all phases of trauma care because discovery of injury may occur dramatically during intubation and mechanical ventilation or may occur surprisingly late in the patient’s posttrauma course. Tracheobronchial tears may also be caused by penetrating injury, frequently in association with esophageal, carotid artery, or jugular vein trauma.
Resuscitation/Critical Care Assessment
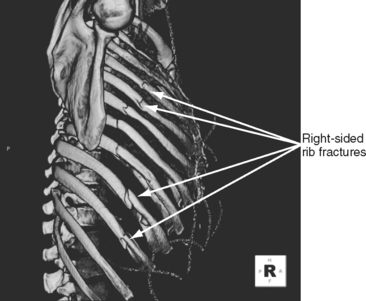
Tracheobronchial injuries are a rare, although often fatal injury, with many patients dying at the scene. In those who survive to reach the hospital, the index of suspicion for tracheobronchial injury is heightened by a history of violent trauma, particularly in patients with fractures of the upper five ribs. The rupture may be immediately symptomatic as evidenced by dyspnea, subcutaneous emphysema, or tension pneumothorax.27 Hoarseness, stridor, and pneumomediastinum may also be present. A tear may be suspected in the patient with mediastinal and subcutaneous emphysema accompanied by a persistent pneumothorax that resists re-expansion. More commonly the rupture develops in two stages. The patient shows almost no symptoms until 3 or 4 days after admission, when pneumothorax or subcutaneous emphysema develops. Should the patient already have chest tubes in place, a persistent pleural air leak is evident, possibly with continued extravasation of air into tissues. Early persistent atelectasis may appear as a result of occlusion of the bronchus with blood and secretions. Bloody secretions are evident on coughing or during suctioning as pleural fluid is drawn back through the damaged airway. These clinical findings require good communication and correlation of nursing and medical observations to make the diagnosis of tracheobronchial trauma. Diagnosis, early or late, is made based on clinical history and the presenting signs and symptoms. CT scanning may suggest injury (presence of mediastinal air), although diagnostic bronchoscopy is still required. Bronchoscopy allows for precise determination of site, nature, and extent of the tracheobronchial defect.43 Presence or even suspicion of the presence of a tracheobronchial injury requires immediate surgical consultation.
Management
Initial management is dependent on the severity of the symptoms described above. A stable patient capable of maintaining the airway and ventilation does not require an immediate artificial airway. Close observation continues as the diagnostic workup proceeds. The patient with significant hemoptysis and airway obstruction must have an airway secured either by insertion of an endotracheal tube or by tracheostomy. Either procedure carries great risk, including further damage to the defect and creation of a false passage. Urgent tracheostomy performed in the operating room may be the safer option.43 A double-lumen endotracheal tube permits the use of independent lung ventilation, thereby facilitating ventilation of the lung with an injured main bronchus with either lower ventilating pressure or straight continuous positive airway pressure (CPAP). If present, pneumothorax, tension pneumothorax, or pneumomediastinum are treated by tube thoracostomy and evacuation of pleural air by suction. The rate and amount of air evacuated by the chest tube are monitored, as is the adequacy of air intake into the lungs. Large air leaks may require a second thoracostomy tube. Immediate thoracotomy is indicated in the presence of massive air leak that prevents adequate ventilation or oxygenation.
Nonoperative management may be considered and implemented in patients with defects that involve less than one third of the circumference of the tracheobronchial tree. Conservative management may result in accumulation of excess granulation tissue that causes airway narrowing. If significant, this may require future long-term surgical intervention.43
Specific Nursing Management
Bronchial injury may be accompanied by lung injury with tears in surrounding small blood vessels, allowing air to enter the pulmonary venous circulation. Monitoring for signs of air embolism is important, particularly before repair or in the patient who is managed conservatively without repair. Sudden cardiovascular deterioration after endotracheal intubation without signs of bleeding may be indicative of air embolism. Focal neurologic signs in the non–head-injured patient are also significant. Placing the patient in Trendelenburg’s position is presumably optimal to trap air in the apex of the left ventricle. Aggressive treatment includes thoracotomy to clamp the pulmonary hilum and access the left ventricle so that a cardiocentesis can be performed to aspirate air bubbles.43 Hyperbaric oxygen therapy may also be used.
Intermediate Care Assessment and Management
Tracheobronchial tears are often diagnosed late in the assessment for occult or missed injury. Patient assessment should focus on identification of posttraumatic complications such as bronchial stenosis. The initial tracheobronchial tear results in a stenosed airway obstructed by granulation tissue and prone to repeated inflammation and infection. Delayed atelectasis appears as granulation tissue obstructs the bronchus. Resection of the area of stricture and reanastomosis may be required to prevent repeated infections and excess scar tissue formation below the level of the stenosis. Laser ablation of stenotic lesions and endobronchial stents are also used.44
Nursing management is directed toward chest physiotherapy for the affected lung areas to facilitate secretion clearance and maintain airway patency. The patient’s vital capacity, chest film, and secretions are monitored. Assistance in coughing is a priority because the injury frequently leaves a residual decrease in bronchial sensitivity resulting in diminished stimuli to initiate a cough.44
POSTTRAUMATIC TRACHEAL FISTULA
Description and Etiology
Tracheoarterial fistula (TAF) (including tracheoinnominate fistula) and tracheoesophageal fistula (TEF) may result from blunt injury. More commonly it is a form of endotracheal or tracheostomy tube trauma. TAF occurs when a fistula forms between the trachea and an artery, usually the innominate artery (also known as the brachiocephalic artery). TEF is a fistula between the trachea and the esophagus. Tube trauma can be a sequela of a difficult intubation or tracheostomy.44 During these procedures a retropharyngeal or tracheal wall nick or tear can occur. The area becomes infected with upper airway secretions, erodes, and becomes a fistula. TAF45 and TEF46 are rare but often fatal complications after tracheostomy or intubation.
Tracheoarterial Fistula
The vessel involved may be the innominate, right carotid, or a lower thyroid artery that has been exposed to pressure from an overinflated cuff or a poorly positioned airway (e.g., a tracheostomy). As the tracheal wall erodes, the vessel is perforated and suddenly bleeds into the airway. The patient may die immediately from hemorrhage through the tracheostomy. Occasionally, sentinel bleeding may appear as hemoptysis. A patient with an artificial airway and hemoptysis warrants notification to a physician and immediate further investigation. Figure 24-11 shows how poor tube positioning and cuff overinflation may cause a hemorrhage. The incidence of TAF in patients with a short- or long-term tracheostomy is similar at 0.7% and is almost always fatal if not recognized and surgically corrected.47
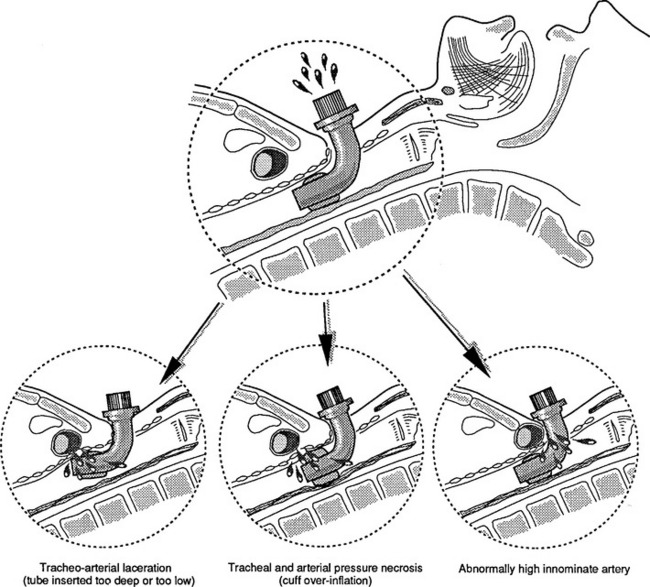
FIGURE 24-11 Tracheoarterial fistula in patients with tracheostomy..
(From Besson A, Saegesser F: Color atlas of chest trauma and associated injuries, vol II, Oradell, NJ, 1983, Medical Economics, p 48.)
Prevention.
Physiologic cuff pressure should be maintained at less than a mean mucosal capillary pressure of 20 to 25 mm Hg. Different commercially available tracheostomy and endotracheal tubes all exert varying tracheal wall pressures, including the soft, large-volume cuffs and foam cuffs, which are autoregulated by an air valve. Proper inflation of the cuff according to product recommendations is useful; however, the actual cuff pressure must be measured. Measurement is made by a purpose-designed cuff pressure manometer or, if necessary, by a simple stopcock and manometer apparatus. If there is reason to believe that the patient is at higher risk for TAF, a shorter or longer tube of appropriate diameter may be inserted to relieve the immediate pressure at the site of tracheal erosion. A pulsating tracheostomy tube is evidence of such risk but does not confirm fistulization. Any patient at high risk can be examined by tracheoscopy for evidence of fistula formation.48
Immediate Management of the Fistula.
An attempt is made by immediate maximal cuff inflation to stem the bleeding. If cuff overinflation fails, the tracheostomy tube is removed, and a translaryngeal tube is inserted. The cuff is again inflated at the level of the fistula to control the bleeding, or the tube may be positioned to allow direct finger pressure for control. The finger is inserted along the anterior trachea and compresses the blood vessel against the sternum. The patient is ventilated and prepared for immediate transport to the operating room. If transport is not possible, the surgical equipment necessary for artery ligation is readied in the patient’s room. Suction must be immediately at hand.45
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree