5 Therapy for Men
 Male Pelvic Floor Function
Male Pelvic Floor Function
There are three functions of the male pelvic floor:
- Support of the abdominal contents
- Elimination of urine and feces
- Sexual function
 Support of the abdominal contents
Support of the abdominal contents
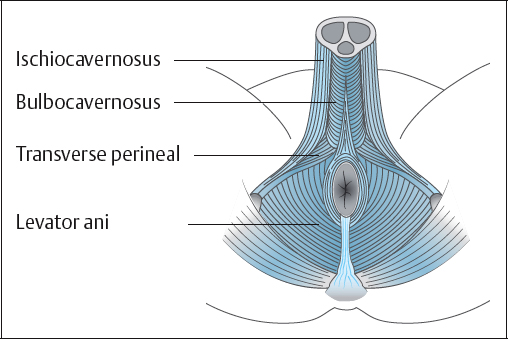
In the male, the pelvic floor muscles extend from the anterior to the posterior of the bony pelvis, forming a diaphragm covering the pelvic outlet which, along with the pelvic fascia, supports the urethrovesical system and rectum (Fig. 5.1).
The periurethral striated muscle is a mixture of fasttwitch and slow-twitch muscle fibers that raise urethral closure pressure during periods of increased intra-abdominal pressure or by voluntary control [DeLancey 1994]. Gosling et al. [1981] reported that the pelvic floor muscles were made up of about two-thirds type 1, slow-twitch, aerobic oxidative fibers, which were continuously tonic to support the pelvic viscera.
Levator ani. The levator ani consists of the pubococcygeus muscle and the iliococcygeus muscle. The pubococcygeus and iliococcygeus muscles, together with the ischiococcygeus muscle, form a muscular diaphragm that supports the pelvic viscera and opposes the downward thrust caused by an increase in intra-abdominal pressure.
Pubococcygeus muscle. The pubococcygeus muscle arises from the back of the pubic bone and the anterior part of the obturator fascia and inserts into a fibromuscular layer between the anal canal and the coccyx. In animals, the pubococcygeus draws the tail under the body.
Iliococcygeus muscle. The iliococcygeus muscle arises from the ischial spine and from the arcus tendineus of the pelvic fascia and is attached to the coccyx and the median raphe. In animals, the iliococcygeus wags the tail.
Ischiococcygeus muscle. The ischiococcygeus arises from the pelvic surface of the ischial spine and is inserted into the side of the coccyx and lower sacrum. It is responsible for pulling the coccyx forward after defecation.
Puborectalis muscle. The puborectalis muscle arises from the pelvic surface of the pubic bone, blends with the levator ani and is inserted into the muscle from the other side, posterior to the rectum, at the anorectal flexure. It can be considered part of the pubococcygeus muscle. It helps maintain fecal continence by maintaining the anorectal angle.
Rhabdosphincter. The intrinsic striated muscle of the external urethral sphincter mechanism in the urethra is called the rhabdosphincter [Dixon and Gosling 1994]. It surrounds the membranous urethra and lies deep to the urogenital diaphragm. It is postulated that it receives triple innervation from the sympathetic, parasympathetic, and somatic nervous systems [Gray 1998]. The superficial muscle fibers arise from the transverse perineal ligament and surrounding fascia and insert into the perineal body. The deep fibers form a continuous circular formation around the membranous urethra. The muscles from both sides together form a sphincter compressing the membranous urethra and assisting in the maintenance of urinary continence.
In a study using histochemical and electronmicroscopic techniques, Light et al. [1997] suggested that the rhabdosphincter consisted of two-thirds slow-twitch fibers and one-third fasttwitch fibers, which enabled the sphincter to maintain urethral closure at rest and during physical activity.
The continence mechanism in men consists of the continuous smooth-muscular structure of the bladder base, the bladder neck, and the proximal urethra, supplemented by the striated muscle fibers of the horseshoe-shaped rhabdosphincter [Elbadawi 1995]. It was Elbadawi who led thinking away from the previous concept of a separate internal and external sphincter, describing instead a “continuous” continence mechanism.
Anal sphincter. The anal sphincter consists of elliptical muscle fibers on each side of the anal canal, attached to the tip of the coccyx posteriorly and inserted into the perineal body anteriorly. Inferiorly, it blends with the skin surrounding the anus; superiorly, it forms a complete sphincter and blends with the puborectalis muscle. The anal sphincter is in a normal state of tonic contraction, but can provide greater occlusion of the anal aperture when needing to contain feces and flatus.
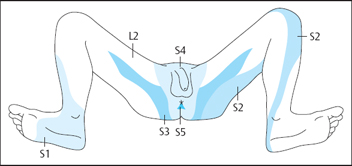
The pelvic floor muscles are supplied by nerve roots S2, S3, and S4.
Bladder storage. Urinary continence relies on three mechanisms: an anatomically intact urinary system; integration of neural modulatory structures in the brain, spinal cord and peripheral nervous system; and a competent urethral sphincter mechanism [Gray 1992, Park et al. 1997]. Maintenance of urinary continence is multifactorial and depends on detrusor control and urethral closure function [Bernstein 1997]. Passive urethral closure is enhanced by the activity of the rhabdosphincter muscle and the use of the pelvic floor muscles, particularly during increased intra-abdominal pressure.
The urine produced by the kidneys is propelled along the ureters into the bladder by peristaltic activity of the smooth ureteric muscle. The viscoelastic bladder is compliant to the volume of urine produced, so that the bladder pressure remains at zero. When the bladder fills to 350–500 mL, the intravesical stretch receptors are stimulated via S2–S4 and trigger a detrusor muscle contraction and a strong desire to void. The first sensation of filling is usually at approximately 200 mL. This initial desire to void can be normally controlled. Voiding will usually take place later, following a stronger desire to void.
 Elimination of Urine and Feces
Elimination of Urine and Feces
Micturition. The external urethral sphincter is relaxed during micturition. At the end of micturition, the sphincter contracts and together with the bulbocavernosus muscle ejects the last few drops of urine.
Voiding is initiated by the relaxation of the striated pelvic floor musculature under voluntary control, and voiding is completed by reflex action. The detrusor muscle contracts, increasing the internal pressure in the bladder. Urine passes through the relaxed involuntary and voluntary muscle of the urethra. Voiding occurs at approximately 15 mL/s in males (20 mL/s in females), although it is faster in young men. Maximum flow rates are obtained at volumes of 300– 400 mL in normal men. Flow rate is improved by a standing position in men [Berger 1995]. Normally, the bladder empties completely.
After micturition, the external urethral sphincter muscles and the pelvic floor muscles (including the bulbocavernosus muscles) contract, while the detrusor muscle relaxes, enabling the bladder to refill, thus repeating the micturition cycle.
Defecation. The external anal sphincter and pelvic floor muscles relax, while the abdominal muscles contract to eliminate feces.
Neurological control of the bladder. The neurological control of the bladder is a complex subject, and the local interactions between neurologic modulators of the urethra and detrusor are less clear [Gray 1992]. The detrusor muscle is under neurological control from the brain, the spinal cord, the peripheral nerves, and the neurotransmitters of the lower urinary tract [Gray 1998].
 Sexual Function
Sexual Function
The two muscles that have an important role in sexual function are the bulbocavernosus and the ischiocavernosus muscles.
Bulbocavernosus muscle. The bulbocavernosus muscle arises from the median raphe and the perineal body. The middle fibers encircle the bulb and corpus spongiosum penis. The middle fibers assist in erection of the corpus spongiosum penis by compressing the erectile tissue of the bulb. The anterior fibers spread out over the side of the corpus cavernosum and are attached to the fascia covering the dorsal vessels of the penis and contribute to erection by compressing the deep dorsal vein of the penis. The bulbocavernosus muscle empties the bulbar canal of the urethra. The fibers are relaxed during voiding and come into action to arrest micturition. Rhythmic contractions of the bulbocavernosus muscle propel the semen down the urethra, resulting in ejaculation.
Ischiocavernosus muscle. The ischiocavernosus muscle arises from the inner surface of the ischial tuberosity and pubic ramus and inserts into an aponeurosis into the sides and under surface of the crus penis. Contractions of the ischio-cavernosus muscles produce an increase in the intracavernous pressure and influence penile rigidity.
 Prostate Conditions
Prostate Conditions
Prostate gland. The prostate gland is a small, walnut-shaped, fibromuscular gland with ducts situated at the base of the bladder surrounding the prostatic urethra. The young adult prostate is about 4 × 3 × 2 cm in size and weighs about 20 g, but the size increases with age [Neal 1997]. The prostate gland produces fluid that provides one of the constituents of semen. The fluid contains nutrients, such as zinc (antibacterial factor) and citrate (sperm transport), enabling sperm motility and mobility [Sant and Long 1994]. Prostate-specific antigen (PSA), a protein secreted by the prostate gland, may be found in the bloodstream. During ejaculation, the smooth muscle of the bladder neck contracts to prevent urine escaping and semen entering the bladder.
 Benign Prostatic Hyperplasia
Benign Prostatic Hyperplasia
Benign prostatic hyperplasia (BPH) is a condition that includes benign prostatic enlargement due to a multiplication of normal cells, lower urinary tract symptoms, and bladder outlet obstruction. The prevalence of benign prostatic enlargement increases with age. However, there is no significant relationship between prostate size and symptoms [Simpson et al. 1996].
Pathologic BPH is divided into two stages: microscopic and macroscopic [Sant and Long 1994]. The earliest microscopic nodules of BPH develop in men between 30 and 50 years of age in the periurethral zone and may infiltrate the transitional zone. The etiology of BPH is imprecisely defined. Aging and the presence of androgens, especially dihydrotestosterone, are essential factors for the development of BPH.
Blockage to the prostatic urethra causes voiding symptoms of hesitancy, weak stream, straining, intermittency, incomplete emptying, and terminal dribble. Severe blockage may cause acute retention of urine and the need for immediate surgery.
Intervention for BPH. Transurethral resection of the prostate (TURP) is the most frequently performed operation for BPH and is carried out using a resectoscope with a cutting loop and coagulating electrode. In all forms of prostatectomy, the bladder neck is resected, rendering the closure mechanism incompetent. Postoperatively, continence relies on the strength and integrity of the external urinary sphincter. Men can experience retrograde ejaculation after prostatectomy. During normal ejaculation, closure of the bladder neck prevents seminal fluid from entering the bladder. However, after surgery, seminal fluid may enter the bladder on ejaculation and is voided during the next micturition.
A retropubic or suprapubic prostatectomy is occasionally carried out for greatly enlarged prostates with BPH. The procedure is carried out through a lower abdominal incision.
The range of minimally invasive treatments for men with BPH has steadily expanded during the last decade. The energy sources range from microwaves and radiofrequency waves to high-intensity focused ultrasound, laser vaporization, coagulation, and resection, and electrosurgical techniques [Djavan et al. 1999]. However, TURP is still regarded as the gold standard surgical option.
As an alternative to surgery, other treatments such as drug therapy and stents may be advised. Patients will be guided by their urologist to make an informed choice from the different treatments available.
There are two drug therapy treatments available for the reduction of urinary symptoms caused by benign prostatic enlargement. Drug therapy using 5a-reductase inhibitors reduces the size of the prostate by inhibiting the enzyme necessary for testosterone metabolism. However, if the medication is withdrawn, the prostate gland will return to pretreatment size or rebound with an increase in size. This medication acts by androgen deprivation and may be responsible for loss of libido in some men.
Alpha-adrenoreceptor antagonists are alpha-blockers that can be used for symptoms of obstruction, due to their relaxant effect on the prostate and internal urethral sphincter. They improve urinary flow rate and reduce day and night frequency. The medication may also have an effect on the intestinal smooth muscle, resulting in constipation. Patients are warned they may have retrograde ejaculation. These alpha-blockers may cause side effects of dry mouth, sedation, dizziness, drowsiness, tachycardia, and palpitations.
For men with retention who are unfit for surgery, wire or silicone mesh stents can be positioned inside the restricted prostatic urethra to allow the free flow of urine. Unfortunately, various problems can arise with the use of stents. They can cause infection and are prone to encrustation or even migration into the bladder.
 Prostate Cancer
Prostate Cancer
Prostate cancer is the second commonest cancer in men in the United Kingdom, after lung cancer [National Statistics Office 2000], and it is the third most common cancer globally after lung and stomach cancer [World Health Organization 2003].
Prostate cancer occurs as the result of primary tumors in the majority of cases [Gray 1992]. Prostate cancer is caused by a multiplication of abnormal cells and is associated with loss of apoptotic potential and uncontrolled proliferation [Denmeade et al. 1996]. Prostate adenocarcinomas originate within the stroma (cortex) of the gland, with a firm, single or multifocal nodule. As the tumor volume increases, it causes enlargement of the prostate gland, which may give rise to symptoms of bladder outlet obstruction. The cause of prostate adenocarcinoma remains unclear, but genetic, racial, viral, and dietary factors have been suggested.
Benign prostatic hyperplasia and prostate cancer are two separate entities. One does not lead to the other, although they may coexist. Prostate cancer may be slow-growing in the elderly, who may die “with” rather than “of” the disease.
Intervention for prostate cancer. Men with prostate cancer may be given the choice of a radical prostatectomy, radiotherapy (known as brachy-therapy if radioactive seeds are used), antiandrogen treatment, or more rarely now orchidectomy. Endocrine therapy includes gonadotropin-releasing hormone antagonists or antiandrogen drug treatment as a treatment for prostate cancer. Side effects include gynecomastia, loss of libido, and erectile dysfunction. Shared decision-making is now considered to be an integral part of gaining informed consent from patients for most urological surgery. Radical prostatectomy is becoming increasingly preferred for localized disease, particularly now that nerve-sparing techniques can help preserve potency and continence [Resnick 1991].
 Urethral Stricture
Urethral Stricture
Urethral stricture occurs when scar tissue narrows the urethra following surgery, urethritis, or trauma. It may occur at any part of the urethra, from the external meatus to the prostatic urethra. At the penoscrotal junction, strictures may form from traumatic catheterization [Denning 1996].
Treatment of a stricture consists of dilation under anesthetic or surgical division by urethrotomy. Maintenance treatment may consist of regular dilation by intermittent self-catheterization [Lawrence and MacDonagh 1988].
 Prostatitis
Prostatitis
Prostatitis may cause considerable rectal and suprapubic discomfort (see pelvic pain, below).
 Male Incontinence
Male Incontinence
Prevalence of urinary incontinence in men. The prevalence of urinary incontinence in men increases with age and ranges from 3.6% in men 45 years old to 28.2% in men 90 years of age or older [Thomas et al. 1980, Britton et al. 1990, Brocklehurst 1993, Malmsten et al. 1997]. The prevalence of reported urinary incontinence in men also varies with the definition of incontinence and the threshold of incontinence used.
The prevalence of urinary incontinence 3 months after transurethral resection of the prostate is about 6% [Emberton et al. 1996, Neal 1997].
The prevalence of urinary incontinence 1 year after radical prostatectomy ranges from 14% to 16% [Davidson et al. 1996, Donnellan et al. 1997].
Classification of incontinence. Incontinence may be classified into stress urinary incontinence, urge urinary incontinence, mixed incontinence, postmicturition dribble, continual incontinence, overflow incontinence, extraurethral incontinence, and functional incontinence.
 Stress Urinary Incontinence
Stress Urinary Incontinence
Stress urinary incontinence is defined as “the complaint of involuntary leakage on effort or exertion, or on sneezing or coughing” [Abrams et al. 2002]. Stress urinary incontinence is almost always iatrogenic. Men at risk are those after radical prostatectomy, radiotherapy, and occasionally transurethral resection of the prostate.
 Urge Urinary Incontinence
Urge Urinary Incontinence
The symptom urge incontinence is “the complaint of the involuntary leakage accompanied by or immediately preceded by urgency” [Abrams et al. 2002].
Urgency. Urgency is “the complaint of a sudden compelling desire to pass urine which is difficult to defer” [Abrams et al. 2002].
Detrusor overactivity incontinence. Detrusor overactivity incontinence is “incontinence due to an involuntary detrusor contraction” [Abrams et al. 2002].
Neurogenic detrusor overactivity. Neurogenic detrusor overactivity is “detrusor overactivity when there is a relevant neurological condition” [Abrams et al. 2002].
Nocturia. Nocturia is “the number of voids recorded during a night’s sleep: each void is preceded and followed by sleep” [Abrams et al. 2002].
Nocturnal polyuria. Nocturnal polyuria is present when an increased proportion of the 24-h output occurs at night (normally during the 8 h when the patient is in bed). The night-time urine output excludes the last void before sleep, but includes the first void of the morning [Abrams et al. 2002]. One definition of nocturnal polyuria is when the nocturnal output of urine is over 35% of the total 24-h urine production [Weiss et al. 1998]. Nocturia increases with advancing age.
Nocturnal enuresis. The symptom nocturnal enuresis is “the complaint of loss of urine occurring during sleep” [Abrams et al. 2002]. The cause remains obscure. Hereditary factors, sleep disturbance and natriuresis (excretion of sodium ions) may play a part, but remain unproven. In children, enuresis may be caused by incomplete bladder emptying. There are similarities between enuresis and nocturia, with both entities being based on nocturnal polyuria, with urine production exceeding the bladder capacity. The absence of a circadian rhythm in arginine vasopressin (AVP) is also associated with both nocturnal enuresis and nocturia [Djurhuus et al. 1999].
 Mixed Incontinence
Mixed Incontinence
The International Continence Society definition of mixed incontinence is “the complaint of involuntary leakage associated with urgency and also with exertion, effort, sneezing or coughing” [Abrams et al. 2002].
 Postmicturition Dribble
Postmicturition Dribble
Postmicturition dribble is the term used “when an individual describes the involuntary loss of urine immediately after he has finished passing urine, usually after leaving the toilet in men” [Abrams et al. 2002]. It should be distinguished from terminal dribble, which occurs at the end of micturition. The amount can be as much as 15 mL. It is a common problem for men of all ages, but particularly troublesome in older men. Postmicturition dribble is attributed to a failure of the bulbocavernosus muscle to evacuate the bulbar portion of the urethra [Feneley 1986, Millard 1987], causing pooling of urine in the bulbar urethra, which can dribble with movement. Men who suffer from postmicturition dribble may wear pads or dribble collectors, or perform bulbar urethral massage [Millard 1987, Pomfret 1993], but pelvic floor muscle exercises are a preferable form of treatment [Paterson et al. 1997, Dorey 2003].
 Continual Urinary Incontinence
Continual Urinary Incontinence
Continual urinary incontinence is the complaint of continuous leakage [Abrams et al. 2002].
 Overflow Incontinence
Overflow Incontinence
Overflow incontinence is any involuntary loss of urine associated with overdistension of the bladder [Abrams et al. 1998]. This may be caused by urethral obstruction or detrusor underactivity.
Detrusor–sphincter dyssynergia. Detrusor– sphincter dyssynergia is defined as “a detrusor contraction concurrent with an involuntary contraction of the urethral and/or periurethral striated muscle.” Occasionally, flow may be prevented altogether [Abrams et al. 2002].
 Extraurethral Incontinence
Extraurethral Incontinence
Extraurethral incontinence occurs rarely when leakage occurs as a result of an ectopic ureter or from a fistula.
 Functional Incontinence
Functional Incontinence
Functional incontinence occurs when men are unable to get to the bathroom in time due to problems of poor mobility and dexterity.
 Assessment of Male Incontinence
Assessment of Male Incontinence
Before a diagnosis can be made, and before treatment can be commenced, a subjective and objective assessment is required [Dorey 2001a].
 Subjective Assessment
Subjective Assessment
The subjective assessment is based on the patient’s account of his symptoms and should include questions in the following categories:
- Patient’s age, occupation, hobbies, and activities
- Chief complaint and other symptoms
- Duration and severity of symptoms
- Amount of leakage
- Frequency of leakage
- Urine stop test (this question provides an opportunity to explain that this exercise can lead to retention of urine, counteracts the normal micturition reflexes, and therefore should not be practiced)
- Bowel activity
- Diet
- Surgical history (dates of transurethral resection of prostate, radical prostatectomy, abdominal surgery, spinal surgery)
- Medical history (including prostatitis, diabetes, latex allergy, metal implants, medications, radiotherapy, neurological problem)
- Previous treatment
- Body mass index
- Sexual problems (difficulty achieving or maintaining penile erection or premature ejaculation)
- Functional factors (mobility and dexterity)
- Motivation
- Medical investigations (urinalysis of midstream urine, uroflow, ultrasound postvoid residual, blood test for prostate specific antigen, urodynamics, cystoscopy, 24-h pad test, frequency/volume chart.
 Objective Assessment
Objective Assessment
The objective assessment includes an abdominal, perineal, neurological, and digital anal examination. The objective assessment can be undertaken by a physiotherapist or nurse with specialist training. The specialist training should include the theory of the assessment and a practical examination of a plastic male model under supervision.
The patient should be given an opportunity to be chaperoned either by a partner or friend or by a member of staff.
The objective assessment should always begin with an explanation of the reasons for needing a digital anal examination. It can be explained that it is necessary to know whether the muscles that help to control continence are working. The strength and endurance of these muscles can be assessed best by feeling them, the method of exercising can be checked, and the correct amount of exercise can be given. The skin sensation can also be checked. If the patient is unhappy about a digital anal examination, he may allow a perineal examination, but he should not be persuaded against his wishes.
Following this detailed explanation, the patient must give informed consent to the objective examination and the consent must be entered in the patient’s notes. At this stage, he should be given the opportunity to visit the lavatory.
For the objective examination, the patient should be lying on his back with two pillows under his head, with his knees bent and his feet on the plinth (the crook-lying position), without his underwear but with a sheet or paper sheet over his pelvis. He may retain his sheath and drainage system if he has one.
Abdominal Examination
In the crook-lying position, the abdomen is palpated for pain, masses that need referral, and bladder distension, which may indicate retention from detrusor underactivity or urethral blockage. This may need training and practice under medical supervision. It may be possible to palpate a ridge marking the extent of a full hard bladder with retention. A hard, swollen abdomen may indicate a distended bladder and the need for immediate referral to a urologist.
Perineal Examination
Initially, it is necessary to observe the pelvic area in the crook-lying position for congenital abnormalities such as hypospadias, in which the urethral meatus opens on the underside of the penis. At this stage, an enlarged testis, warts, hemorrhoids, and tumors may be identified. The skin condition should be examined for evidence of redness, infection, and excoriation in the penile, perineal, scrotal, and anal areas.
The patient may then be asked to tighten the anus as if to prevent wind escaping, while the anal wink is observed. Then he can be asked to tighten at the front as if to prevent the flow of urine and feel a scrotal lift and the base of the penis pull back toward the abdomen. After this, he is asked to give an unguarded cough, which may provide evidence of leakage. He is then requested to cough while he is tightening his pelvic floor muscles to prevent leakage, which may provide evidence of urinary control.
The S4 dermatome (Fig. 5.2) can be tested by using a cotton wool ball or a gloved finger by stroking gently either side of the anus and either side of the perineum while asking the patient if it feels the same on both sides. If there is neurological deficit, the S2 dermatome can be checked on the lateral surface of the buttock, lateral thigh, posterior calf, and plantar heel, and the S3 dermatome can be checked on the upper two-thirds of the inner surface of the thigh. If neurological impairment is suspected, the bulbocavernosus reflex can be tested during the digital anal examination. The patient should be pre-warned. Gentle pressure on the glans penis during a digital anal examination elicits an anal sphincter contraction unless there is neurological impairment.
Digital Anal Examination
The patient is placed in the crook-lying position. The therapist approximates a gloved index finger, covered amply with lubricating gel, to the anal meatus, allowing the patient to feel the gel. The patient is then asked to bear down on to the finger as if he is letting wind escape. While the patient is bearing down, the finger is inserted straight, in a cephalad direction (toward the head), with the finger pad toward the coccyx. The finger can then be introduced to 1–2cm from the meatus, and the integrity and tone of the external anal sphincter can be felt [Dixon et al. 1997]. Any areas of pain should be noted. With a lax sphincter, it may be possible to feel areas of scar tissue in the external anal sphincter where there is no muscle contraction. The patient should be asked to contract the anus and hold for 5 s, while the therapist grades the strength of the contraction and notes the duration of the hold. This can be repeated twice and then the ability to perform a fast contraction noted. The examining finger can then be introduced to 3–4cm from the meatus and the anterior pull of puborectalis gently felt. This muscle is then graded, as for any voluntary muscle in the body—0–5 for muscle strength, for the duration of the hold, and for the ability to perform a fast contraction. From this digital anal examination, the anal sphincter and the puborectalis can then be assessed and recorded using the modified Oxford scale: 0 = nil, 1 = flicker, 2 = weak, 3 = moderate, 4 = good, 5 = strong, 6 = very strong [Dorey 2003].
Diagnoses
The patient should be informed of the findings from the digital anal examination. The pelvic floor strength (0—6), the length of hold up to 10 s (endurance), the number of repeats, and the ability to perform a fast contraction should be discussed and recorded.
After the subjective and objective assessment, it is possible to make a problem list (multiple diagnoses), detail the aims of treatment, list the treatment modalities to be used, make a note of any advice to be given, and form a treatment plan.
 Intervention for Male Incontinence
Intervention for Male Incontinence
Pelvic floor muscle exercises, biofeedback, bladder training, electrical stimulation, behavioral strategies and advice have all been used in the treatment of men with lower urinary tract symptoms [Dorey 1998, 2000a, 2001a] (Table 5.1). In a literature review, Moore and Dorey [1999] found that the benefits in men were not well researched, although in nonrandomized and uncontrolled trials, the results appeared encouraging [Sotiropoulus et al. 1976, Krauss and Lilien 1981, Burgio et al. 1989, Meaglia et al. 1990, Jackson et al. 1996, Mathewson-Chapman 1997, Chang et al. 1998].
| Classification | Symptoms | Treatment options |
|---|---|---|
| Urge urinary incontinence (hyperactive bladder) | Involuntary loss of urine associated with a strong desire to void; may be due to detrusor overactivity |
|
| Stress urinary incontinence | Involuntary loss of urine on physical exertion or increase in intra-abdominal pressure due to sphincter deficiency |
|
| Mixed incontinence (urge and stress) | Urge and stress symptoms | – Combination of the above |
| Post-micturition dribble | Loss of urine after micturition due to urine retention in the bulbar part of the urethra |
|
| Postprostatectomy incontinence | Symptoms of urge and/or stress urinary incontinence and/or post-micturition dribble |
|
| Overflow incontinence | Involuntary loss of urine associated with overdistension of the bladder due to detrusor underactivity or bladder outlet obstruction or detrusor–sphincter dyssynergia |
|
| Functional incontinence | Due to functional problems exacerbating existing bladder problems—e. g., poor mobility, difficult clothing, confusion |
|
 Stress Urinary Incontinence
Stress Urinary Incontinence
Stress urinary incontinence in men may occur due to sphincter damage following a prostatectomy [Donnellan et al. 1997]. The internal sphincter is damaged in all forms of prostatectomy. Physiotherapy has the potential to be effective in alleviating stress incontinence caused by an incompetent urethral sphincter, through the use of pelvic floor muscle exercises.
Pelvic Floor Muscle Exercises for Stress Urinary Incontinence
The passive urethral mucosal seal is compressed actively by the external urethral sphincter and pelvic floor muscles [Harrison and Abrams 1994]. Pelvic floor muscle exercises are noninvasive and are not associated with serious complications [Gray 1992]. Pelvic floor muscle exercises should be individually taught, to ensure that the patient is lifting up the pelvic floor and not bearing down as if defecating (i. e., performing a Valsalva maneuver). The amount and progression of patient-specific pelvic floor exercise is determined by individual assessment and digital anal examination. Men can be encouraged to tighten and lift the pelvic floor muscles, as in the control of flatus or the prevention of urine flow, and can practice in front of a mirror to observe a visible withdrawal of the penis and a scrotal lift [Paterson et al. 1997, Moore et al. 1999]. Patients can be taught to palpate a contraction of the ischiocavernosus muscle at the perineum 2cm medially and 2cm anteriorly to the ischial tuberosity.
The convenient positions for practicing pelvic floor muscle exercises are in the crook-lying position with the knees bent or apart; standing with the feet apart; and sitting with the knees apart [Burgio et al. 1989, Moore et al. 1999, Paterson et al. 1997, Van Kampen et al. 2003]. It is the intensity rather than frequency of work that is important, as maximal voluntary effort causes muscle hypertrophy and increased muscle strength [Guyton 1986, DiNubile 1991, B⊘ 1994]. In order to achieve full fitness, pelvic floor muscle exercises should be taught for endurance as well as for muscle strength by submaximal contractions [Guyton 1986]. Muscle training, therefore, depends on the motivation of the patient and the adherence to the pelvic floor exercise regimen [Jackson et al. 1996]. Patients may find it helpful to keep an exercise diary.
Muscle strength development is achieved by a combination of the recruitment of a greater number of motor units, a higher frequency of excitation, and muscle hypertrophy. A contraction as close to maximum as possible is required to create high tension, in order to address the principles of overload and specificity [DiNubile 1991, B⊘ 1994]. In order to achieve full fitness, pelvic floor muscle exercises should be taught for endurance as well as for muscle strength.
“The knack” is the ability to initiate a pelvic floor muscle contraction sufficiently far in advance of an intra-abdominal pressure rise [Ashton-Miller and DeLancey 1996]. It is called “the knack” in recognition of the motor control skill required.
Pelvic floor muscle exercises should be patient-specific. The hold time in seconds is ascertained from the digital anal assessment. The rest time should exceed the hold time, to allow muscle fiber recovery. There is no evidence for an optimum number of repeat contractions, but ongoing objective assessment will help determine what is appropriate for each patient. The quality of contraction is more important than the quantity. Exercises should be practiced every day. A typical program practiced twice a day could be: three maximal contractions in the crook-lying position, three maximal contractions in a sitting position, and three maximal contractions standing, held for up to 10s. However, this is only a guide. Some contractions can be activated quickly and some slowly. The patient can also be encouraged to lift the pelvic floor up to 50% of the maximum while walking, to encourage the supporting role of the pelvic floor muscles. Men can be taught “the knack” of tightening the pelvic floor muscles before activities that increase intra-abdominal pressure, such as coughing, sneezing, rising from a seated position, or lifting [Ashton-Miller and DeLancey 1996].
Specific Home Exercise Program
No research has been conducted to provide clear indications of the number of pelvic floor muscle exercises that should be carried out in order to build up muscle bulk, strength, and endurance. Dorey [2003] recommended maximum-strength home exercises twice a day in the crook-lying, sitting, and standing positions, and included functional work, and reported considerable improvement. In order to gain compliance, it is surely better to have an honest agreement with the patient and let him know the exact number of contractions required.
Biofeedback Treatment for Stress Urinary Incontinence
Biofeedback is a mechanism by which the patient is more aware of pelvic floor muscle activity and encourages greater muscular effort [Burgio et al. 1989, Knight and Laycock 1994, Jackson et al. 1996, Van Kampen 2003]. Many patients are unable to contract the pelvic floor, or do not understand the maximum effort needed. Biofeedback can often provide the necessary awareness for muscle reeducation. There are three recognized methods of biofeedback: digital, manometric (pressure), and electromyographic [Bump et al. 1991, Haslam 1999]. The three biofeedback methods have been studied and compared, and they appeared to correlate with each other well [Haslam 1999].
Digital anal biofeedback. A lubricated and gloved index finger can be used during a digital anal examination to monitor the strength and endurance of the external anal sphincter and the puborectalis muscle. This information can then be communicated to the patient in order to provide feedback and encouragement.
Manometric biofeedback. An anal pressure probe can be used to monitor muscle activity and provide manometric biofeedback. The anal probe can be attached to a manometer with a visual display. Sophisticated computerized equipment with a colored visual display screen, audible feedback, a variety of work and rest programs, and a printer is considered the gold standard by many therapists. This equipment can also be used for electromyographic biofeedback and electrical stimulation. The patient should be placed in the crook-lying position with the knees bent and apart, with a sheet or paper sheet over his pelvis. He should be able to see and hear the monitor screen. The probe should be covered with a condom, lubricated with gel, and approximated to the patient’s anus. The patient is then asked to bear down as if releasing flatus, while the probe is gently inserted. The probe needs to be held in a position that records maximal readings and prevents it from slipping out.
The manometric pressure is usually measured in bars or numerical units using anal perineometry, or by centimeters of water (cmH2O) or millimeters of mercury (mmHg) using more sophisticated computerized equipment.
Electromyographic biofeedback. Electromyography (EMG) is the study of minute electrical potentials produced by depolarization of the muscle membrane [Siroky 1996]. Bioelectric activity of the muscles can be recorded using either a needle sensor, an anal probe, or surface sensors. The bioelectric activity is measured in units of microvolts (mV).
The anal probe is easier to apply using lubricating gel whilst the patient bears down as if to release wind, but most probes need to be held by the therapist to prevent slippage. New-shaped anal electrodes fit snugly into position and allow ambulatory use. Anal probes are strictly for single-patient use. The anal probes are attached to a computerized biofeedback machine, which monitors the bioelectric activity of the muscles by EMG biofeedback and provides a colored visual display and sometimes an audible signal to encourage greater effort.
Surface skin sensors may be used on the perineum. They can be used to monitor the activity of the pubococcygeus and the ischiococcygeus muscles. The skin needs to be cleansed with an alcohol swab to remove any oils that may prevent good contact. It may be necessary to shave the area. Better contact is made by using new surface sensors for each treatment. Two small, sticky surface sensors can be placed longitudinally over the pubococcygeus and the ischiococcygeus muscle to be monitored by EMG and can be placed 1 cm lateral to the midline. A third sensor can be placed over a bony point such as the sacrum or coccyx, to act as a reference (grounding) to cut out extraneous “noise” from the electrical activity of other muscles in the area. However, some EMG equipment uses a triangular arrangement for all three sensors, with the two active sensors longitudinal to the muscle fibers. One of the benefits of EMG is its use in functional positions. Problems associated with the use of EMG may be due to the size of the sensor pad or probe, which may pick up electrical activity from the surrounding muscles. Surface EMG is noninvasive, painless, and can also be used as an initiator of cerebral control, a tool of assessment, a motivator, and a method of recording; it can be used both to encourage and challenge the patient [Haslam 1998]. Manometric or EMG biofeedback is a useful adjunct to pelvic floor muscle reeducation to stimulate greater patient effort.
Electrical Stimulation for Stress Urinary Incontinence
Electrical stimulation has been used for men with stress urinary incontinence postprostatectomy [Sotiropoulos et al. 1976, Kraus and Lilien 1981, Hirakawa et al. 1993, Bennett et al. 1997, Moore et al. 1999].
Maximal electrical stimulation can be applied pulsed at the maximum intensity tolerable for short periods of 20 min at a frequency of about 30–50 Hz to produce a tetanic contraction. The pulse train off-time should exceed the on-time in order to prevent muscle fatigue. Knight and Laycock [1994] stated that a pulse width of 200 ms produced excitation at relatively low current intensity and was more comfortable than shorter pulse widths. They suggested that acute maximal electrical stimulation may benefit patients with very weak pelvic floor muscles.
Electrical stimulation of the pelvic floor muscles can be delivered by anal or surface electrodes. The anal electrode contains positive and negative bands and is used alone. It is strictly for single-patient use. Surface electrodes may be placed on the coccyx and the perineum or on either side of the perineal body. Care should be taken to avoid burning, which may occur with small electrodes. Skin sensation should be tested before treatment, and the skin should be viewed after treatment. Contraindications to electrical stimulation are listed in Table 5.2.
A current of sufficient amplitude will excite nerve and muscle tissue in its field, causing a muscular contraction. Electrical stimulation has been used for patients who are initially unable to contract the pelvic floor muscles.
The real clinical benefit of electrical stimulation remains to be clarified. It may be that it can increase the circulation to the pelvic floor, or it may be useful for showing patients how to initially contract the pelvic floor muscles.
|
 Urge Urinary Incontinence
Urge Urinary Incontinence
The filling symptoms of frequency, nocturia, urgency, and urge urinary incontinence can be treated with pelvic floor muscle exercises, behavioral training, and lifestyle changes, including fluid intake advice [Burgio et al. 1989, Paterson et al. 1997, Dorey 1998, Van Kampen 2003].
Pelvic floor muscle exercises for urge urinary incontinence. Pelvic floor muscle exercises can be used for urge urinary incontinence to strengthen the pelvic floor musculature and restore the ability to control the urge to void urine. It is suggested that when the pelvic floor contracts, the detrusor muscle will relax due to the activity of the perineopudendal facilitative reflex [Mahony et al. 1977]. Muscle training can be enhanced by the use of biofeedback, as discussed in the treatment for stress urinary incontinence (see above).
Electrical stimulation for urge urinary incontinence. Urge urinary incontinence can be treated with continuous or pulsed low-intensity biphasic electrical stimulation at a frequency of 5–10 Hz, with a pulse width of 200 ms, for up to 20 min for urge suppression [Fall and Lindstrom 1991, Jones 1994]. Geirsson and Fall [1997] used stimulation parameters of 0.75 ms continuous biphasic waves with a frequency of 5 Hz in the treatment of overactive bladder, in order to cause inhibition of detrusor contractions.
Lifestyle changes and behavioral techniques for urge urinary incontinence. There are several non-invasive techniques which, singly or in combination, can improve the symptoms of frequency, nocturia, urgency, and urge urinary incontinence. These include bladder retraining, treating constipation, weight reduction, the adjustment of fluids, and the review of medications, including diuretics. In collaboration with other members of the health-care team,bowelmanagement,weightloss, medication review and treatment of urinary tract infection may all improve symptoms. Education, attention to the quantity, type, and timing of fluid intake, avoiding constipation, and delaying the urge to micturate are now considered part of lifestyle changes that can alter previous behavior patterns. Due to research limitations, current knowledge and practice are based on expert opinion and consensus, but not on strong evidence.
Bladder training for urge urinary incontinence. Bladder training [Frewen 1979] or urge suppression is a method of consciously suppressing the urge to void in order to delay micturition and increase functional bladder capacity [Wells 1988]. Deferment techniques to delay voiding can be taught with strategies such as keeping calm, sitting on a hard surface, standing still, waiting for 1 min for the urge to disappear, and distraction. Once the urge has abated, men will be able to continue their activities, thus allowing their bladder to stretch and fill further. If they wish to visit the bathroom, they should never dash mid-urge. Patients undergoing bladder training need considerable encouragement, motivation, and determination to succeed.
Fluid intake. Guyton [1986] stated that normal fluid intake averages 2300 mL per day, two-thirds of which (1518 mL) is direct fluid, while the remainder is a product of food synthesis. It may be more helpful to monitor the 24-h urine output, which should be about 1000–1500 mL. Normal intake should be increased during hot weather, with strenuous activity, and when eating salty foods. No one should ever be thirsty. If the urine is dark, the patient should be encouraged to drink more fluid. Water is the best thirst quencher.
Diuretics. Natural diuretics are commonly xanthines, such as caffeine and theobromine, occurring primarily in beverages, which act chemically in the body to increase urine production. Caffeine occurs naturally in about 60 species of plants, most commonly in coffee beans, tea leaves, cocoa seeds, and the cola nut [Moore 1990]. Caffeine is also added to several over-the-counter medicines, to counteract the drowsiness that the drug produces as a side effect or to enhance analgesic absorption [Wells 1988]. Caffeine is a bladder irritant and stimulant, with a diuretic effect. It can cause the detrusor muscle to contract, with implications of nocturia, frequency, urgency, and urge urinary incontinence [Addison 1997]. Caffeine should be reduced slowly over a 3-week period in order to prevent withdrawal symptoms of headaches or drowsiness.
Cranberry juice. In the 19th century, the North American Indians used crushed cranberries as a herbal remedy for the treatment of urinary tract infections [Bodel et al. 1959, Moen 1962]. It is also believed that cranberry juice has a bacteriostatic effect by affecting the adherence of certain organisms, particularly Escherichia coli, to the lining of the bladder [Beachy 1981].
Addison [1997] recommended cranberry juice for patients with a high risk of urinary tract infection, those with cystitis from E. coli, patients with indwelling catheters, those undertaking intermittent self-catheterization, and those using sheath drainage. He considered that the recommendation of cranberry juice should be supported by written patient information and be monitored and recorded with dosage, patient instructions, contraindications, side effects, and expected outcomes. There is controversy regarding the drinking of cranberry juice, as drinking in excess of 1 L a day over a prolonged period may increase the risk of uric stone formation [Rogers 1991]. Other side effects include gastritis, increased joint pain for rheumatoid arthritis sufferers [Addison 1997], and diarrhea in patients with irritable bowel syndrome [Leaver 1996].
Medication for urge urinary incontinence. For men with severe urge urinary incontinence and men with nocturnal enuresis, anticholinergic medication may be helpful whilst they are receiving conservative treatment. The side effects include a dry mouth, drowsiness, constipation, and vision accommodation difficulties.
 Postprostatectomy Incontinence
Postprostatectomy Incontinence
Postprostatectomy incontinence should be treated according to the presenting symptoms. Following TURP and radical prostatectomy, patients may have stress urinary incontinence due to sphincter damage, urge urinary incontinence, or a combination of both types. The internal urethral sphincter at the bladder neck is damaged in all forms of prostatectomy and continence relies on a competent external urethral sphincter, reinforced by the pelvic floor musculature.
Preprostatectomy intervention. It would be useful for patients to receive pelvic floor muscle exercise education when they are fit before prostate surgery. They could then increase the strength and endurance of the pelvic floor muscles to help prevent or reduce incontinence postsurgery [Porru et al. 2001].
Postprostatectomy intervention. After TURP and after radical prostatectomy, patients can carry out pelvic floor muscle exercises when the catheter has been removed. Some consultants allow gentle pelvic floor exercises whilst the catheter is in place. After surgery, patients may have iatrogenic stress urinary incontinence due to sphincter damage, or urge urinary incontinence, or a combination of the two types. Symptoms of frequency, nocturia, urgency, urge urinary incontinence, stress urinary incontinence, and postmicturition dribble can be treated conservatively. Treatment modalities include pelvic floor muscle exercises, biofeedback, electrical treatment, lifestylechanges, and bladder retraining. “The knack” of tightening at times of increased intra-abdominal pressure should be taught, to help patients cope with activities such as moving from sitting to standing and with coughing and sneezing [Ashton-Miller and DeLancey 1996].
 Postmicturition Dribble
Postmicturition Dribble
Research has shown that pelvic floor muscle exercises are extremely effective for postmicturition dribble and should be advised in preference to bulbar urethral massage (urethral milking) [Dorey 2003]. In a randomized single-blind trial to test the efficacy of urethral milking, Paterson et al. [1997] found that pelvic floor muscle exercises were almost twice as effective as urethral milking, and the authors recommended pelvic floor exercises as a treatment for this condition. Contracting the pelvic floor muscles after voiding may facilitate a contraction of the bulbocavernosus muscle, which acts to eliminate urine from the bulbar portion of the urethra. This technique is termed a “squeeze-out” pelvic floor muscle contraction [Dorey 2003].
 Overflow Incontinence
Overflow Incontinence
Clean intermittent self-catheterization is used for patients with incomplete emptying due to detrusor underactivity or detrusor–sphincter dyssynergia. Detrusor underactivity occurs when the smooth detrusor muscle fails to contract, due to either lack of neurological control or overstretching. Overstretching may also cause pelvic nerve compression. The detrusor contraction may not be strong enough to empty the bladder completely. Detrusor–sphincter dyssynergia is due to neurological impairment and occurs when the detrusor muscle and sphincter contract simultaneously whilst voiding. This can result in retention of urine in the bladder.
 Neurological Detrusor Overactivity Incontinence
Neurological Detrusor Overactivity Incontinence
This is the result of a neurological condition. The bladder fills and empties automatically by detrusor overactivity. The treatment of this type of incontinence consists of clean intermittent self-catheterization, a sheath drainage system, or—as a last resort—an indwelling catheter. For patients who are permanently catheterized, a suprapubic catheter is more acceptable. Medication, in the form of anticholinergic drugs, or surgery, such as a clam ileocystoplasty, sphincterotomy for detrusor dyssynergia, or sacral root stimulation by neuromodulation, may be necessary.
 Extraurethral Incontinence
Extraurethral Incontinence
Extraurethral incontinence occurs when the distal end of the ureter opens into a place other than the bladder, such as the rectum or perineum. A fistula—a passageway connecting the bladder to the bowel, resulting from trauma or infection following surgery—will also cause extraurethral incontinence. Patients with extraurethral incontinence need to be referred for surgery.
 Functional Incontinence
Functional Incontinence
Functional incontinence is incontinence that is caused by problems of poor mobility and dexterity. Patients may find it difficult to reach the toilet in time, due to physical and environmental factors. Functional incontinence should be treated by improving the patient’s environment, by social care and aids, and by lifestyle and clothing adaptations.
 Treatment Outcomes
Treatment Outcomes
Treatment aims to achieve urinary control, continence, and confidence by conservative measures. However, it is not always possible to achieve this outcome, and patients may need help with managing their condition. It is important to set realistic goals.
 Pelvic Pain
Pelvic Pain
Pain syndromes in the urogenital area have been well described, but are underrecognized and poorly understood [Wesselmann et al. 1997]. They include orchialgia, penile pain, prostate pain, and perineal pain. Wesselmann et al. [1997] stated that some pain relief can be provided by a multidisciplinary approach using pain medications, local treatment regimens, physiotherapy, and psychological interventions.
 Orchialgia
Orchialgia
Orchialgia is pain in one or both testicles. Hayden [1993] and Holland et al. [1994] treated orchialgia with transcutaneous electrical nerve stimulation (TENS) and considered the treatment beneficial.
 Penile Pain
Penile Pain
Penile pain may be caused by penile prosthesis surgery, intracavernosal injections and circumcision, and may have a psychological aspect [Wesselmann et al. 1997]. There have not been any psychological studies of penile pain independently of other urogenital pain disorders. Causative conditions are paraphimosis, Peyronie’s disease, priapism, and herpes genitalis. Penile pain may be relieved if the underlying cause is treated [Gee et al. 1990].
 Prostatitis
Prostatitis
Prostatitis occurs as a result of inflammation of the glandular portion of the prostate [Gray 1992], causing discomfort in the rectal and suprapubic areas. The semen may be yellow or blood-stained. There are four types of prostatitis: acute bacterial, chronic bacterial, nonbacterial (prostatosis) and nonbacterial prostatodynia. Acute and chronic bacterial prostatitis occur as a result of bacteria ascending via the urethra. The causal agents in nonbacterial prostatitis and prostatodynia have not been identified.
The symptoms of prostatitis may be malaise and fever in the acute stage before the onset of dysuria, urgency, frequency, and obstructive voiding. In both the acute and chronic stages, there may be pelvic pain.
Treatment for both bacterial and nonbacterial prostatitis is modestly effective. Antibiotics are used for bacterial prostatitis. Nonbacterial prostatitis has been treated with pulsed short-wave therapy [Singh et al. 1997]. However, pseudodyssynergia has been misdiagnosed as chronic prostatitis [Kaplan et al. 1997].
 Prostatodynia
Prostatodynia
Prostatodynia is nonbacterial inflammation of the prostate and urethra. Pelvic floor relaxation techniques and short-wave therapy have been used for men with prostatodynia [Segura et al. 1979, Singh et al. 1997].
 Perineal Pain
Perineal Pain
Perineal pain may be present in patients with orchialgia and prostatodynia [Wesselmann et al. 1997]. Myofascial release may help in the management of pain from pelvic dysfunction.
 Cancer Pain
Cancer Pain
It is important to note that there are no symptoms with prostate and bladder cancer in the early stages. The first sign of bladder cancer is hematuria. No two cancers are the same. In the later stages of prostate cancer, there are symptoms of hip, leg, and back pain, which may be mistaken for arthritis.
 Proctalgia Fugax
Proctalgia Fugax
Proctalgia fugax causes anal pain due to spasm of the anal sphincter. This can sometimes be alleviated by sitting on the toilet and bearing down as if voiding feces, thereby relaxing the anal sphincter.
 Erectile Dysfunction
Erectile Dysfunction
 Introduction
Introduction
Definition. Erectile dysfunction was defined by a National Institutes of Health Consensus Development Conference in 1993 as “the inability to achieve or maintain an erection sufficient for satisfactory sexual performance” [National Institutes of Health 1993].
Prevalence. An estimated 152 million men worldwide suffered from erectile dysfunction in 1995, and this figure was projected to rise to 322 million men worldwide in 2025 [Aytac et al. 1999]. Feldman et al. [1994] found that the problem was strongly age-related and reported that the probable prevalence of complete or partial erectile dysfunction rose from 40 % in 50-year-olds to 66% in 70-year-olds. However, Lewis and Mills [1999] stated that age-related diseases may be the risk factors for erectile dysfunction, rather than age itself. With increased life expectancy and with a growing population, the number of men with erectile dysfunction will be increasing.
Severity. There are varying degrees of erectile dysfunction; mild erectile dysfunction has been described as achieving a satisfactory erection in seven or eight attempts out of 10; moderate erectile dysfunction as achieving erection in four to six attempts out of 10; and severe erectile dysfunction as achieving an erection in zero to three attempts out of 10 [Albaugh and Lewis 1999].
Causes of erectile dysfunction. Erectile dysfunction may have arteriogenic, venogenic, diabetic, neurological, psychological, drug-induced, and trauma-related causes. Risk factors include accidental trauma, trauma from surgery, and radiation therapy [Lewis and Mills 1999]. Lifestyle-related factors include cigarette smoking [Dorey 2001b], chronic obstructive lung disease, alcohol abuse [Gambert 1997, O’Farrell et al. 1998, Tan and Philip 1999], drug abuse [Benet and Melman 1995], bicycling [Andersen and Bovim 1997, Nayal et al. 1999], and horse riding [Albaugh and Lewis 1999].
Erectile dysfunction always has a psychological component in addition to the underlying cause [Intili and Nier 1998].
Research has shown that erectile dysfunction can be caused by weakness of the bulbocavernosus and ischiocavernosus muscles [Claes and Baert 1993, Colpi et al. 1994, Ballard 1997, Van Kampen 2003, Colpi et al. 1999, Mamberti-Dias et al. 1999, Dorey 2003].
Anatomy of the penis. The penis consists of three cylindrical erectile bodies: dorsally, the two corpora cavernosa communicate with each other for three-quarters of their length, and ventrally the corpus spongiosum surrounds the penile portion of the urethra. The proximal end of the corpus spongiosum forms a bulb attached to the urogenital diaphragm and at the distal end expands to form the glans penis [Kirby et al. 1999].
Muscles associated with penile rigidity. The muscles influencing penile rigidity are the ischiocavernosus and the bulbocavernosus muscles. The ischiocavernosus muscle is a paired muscle that arises from the medial aspect of the ischial tuberosity and inserts into the medial and inferior surface of the corporal bodies. The bulbocavernosus muscle originates from the central perineal tendon and attaches to the dorsal surface of the corpus spongiosum and to the fascia surrounding the corpora cavernosa that covers the deep dorsal vein of the penis. Contractions of the ischiocavernosus and bulbocavernosus muscles produce an increase in the intracavernous pressure. Rhythmic contraction of the bulbocavernosus muscle propels the semen down the urethra, resulting in ejaculation. Both muscles are supplied by the perineal branch of the pudendal nerve (S3-S4).
Mechanism of erection. Penile erection occurs following a series of integrated vascular events, culminating in the accumulation of blood under pressure and end-organ rigidity [Moncada Iribarren and Saenz de Tejada 1999]. This vascular process can be divided into five phases:
- Flaccidity. A state of low flow of blood and low pressure exists in the penis.
- Latent or filling phase. When the erection mechanism is initiated by any stimulus, the penile smooth arterial muscle relaxes and the cavernosal and helicine arteries dilate, enabling blood to flow into the lacunar spaces.
- Tumescence. The venous outflow is reduced by the compression of the subtunical venules against the tunica albuginea (corporal venoocclusive mechanism), causing the penis to expand and elongate, but with a scant increase in intracavernous pressure.
- Full erection. The intracavernous pressure rapidly increases.
- Rigidity. The intracavernous pressure rises higher than the diastolic pressure and blood inflow occurs with the systolic phase of the pulse, enabling complete rigidity to occur. Contraction or reflex contraction of the ischiocavernosus and bulbocavernosus muscles produces changes in the intracavernous pressure. No arterial flow occurs.
- Detumescence. Contraction of the penile smooth muscles and contraction of the penile arteries leads to a decrease of blood in the lacunar spaces, and the contraction of the smooth trabecular muscle leads to a collapse of the lacunar spaces.
 Treatment of Erectile Dysfunction
Treatment of Erectile Dysfunction
There are a range of treatments for erectile dysfunction, including pharmacotherapy, injectables, topical creams, intraurethral drugs, vacuum devices, constriction bands, counseling, pelvicfloor muscle exercises, biofeedback, electrical stimulation, and electroacupuncture.
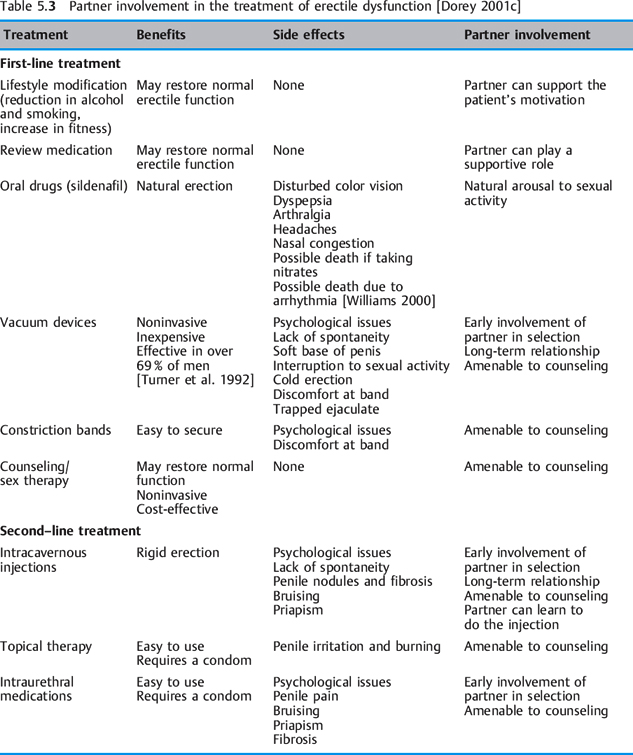
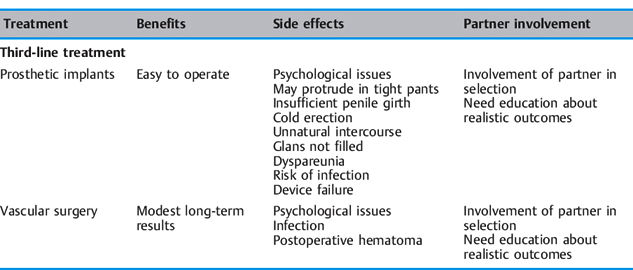
From a review of the literature, a table has been compiled indicating the possible partner involvement for each of the current first-line, second- line, and third-line treatments for erectile dysfunction [Dorey 2001c]. Patients and their partners preferred less invasive forms of treatment [Jarrow et al. 1996] (Table 5.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree