3 Theoretical considerations in the clinical application of WBV to sarcopenia, osteoporosis and metabolic syndrome
Whole body vibration (WBV) has been advocated as a training regimen for improving power in athletes and functional sit to stand, ambulatory, reaching and balancing capacity in the elderly. WBV has also been advocated as a therapeutic tool for improving several conditions including osteoporosis and sarcopenia. Although WBV is becoming more frequently used in gyms and among athletes, the effects on the musculoskeletal system have been reported most significantly in the older population and in those who could be considered as deconditioned or vulnerable, such as people undergoing prolonged bed rest or space flight. Because of the relatively recent development of WBV, some of the research supporting WBV is tenuous at best. Hence, this chapter will explore some of the theoretical possibilities and sites of action of WBV in diseases such as osteoporosis, sarcopenia and metabolic syndrome.
The majority of the literature examines the effect of WBV on muscle function. As stipulated previously, strengthening regimens using WBV have proposed that the mechanism acts purely on neurophysiological reflex mechanisms induced in the muscles, skin and joint receptors. Additional to these mechanisms, theories of tensegrity (Ingber 2006, Sultan et al 2004) suggests that direct mechanotransduction of vibration to ‘cell–cell adhesion molecules’ and unconventional myosin motors at a cellular cytoskeletal level are also likely to have a significant impact on the pre-stress and hence health of muscular tissue through the additional influx of mechanical kinetic and potential energy which the WBV imparts. Furthermore, theories from tensegrity may have an important bearing on explaining the effect of WBV on trabecular bone function.
Sarcopenia
The loss of muscle mass commencing in the fourth decade of life is termed sarcopenia (Morley et al 2001). This has significant consequences on morbidity and vitality as skeletal muscle contains 50–75% of the human body’s proteins and represents a store of energy and nitrogen which become a vital supply of fuel for the immune system, as well as a substrate for wound healing during malnutrition, injury and disease (Rasmussen & Phillips 2003). Lean muscle mass accounts for 90% of the cross-sectional area in active young men, but only 30% of that area in frail older women (Rosenberg & Roubenoff 1995). The prevalence of clinically significant sarcopenia is estimated to range from 8.8% in ‘young old’ women to 17.5% in ‘old old’ men (Morley et al 2001). Sarcopenia has far-reaching consequences for people’s health including:
• increased morbidity due to increased incidence/risk of falls;
• metabolic syndrome (diabetes, high blood pressure, hyperlipidaemia, heart disease and obesity); and
Muscle atrophy is the first step along a cascade of events leading to sarcopenia. Whereas muscle atrophy is reversible, sarcopenia is considered by most people as non reversible. In turn sarcopenia leads to reduced mechanical loading of bones which generates osteopenia while body fat content is increased through the consequential reduction in basal metabolic rate (BMR). Since muscle mass is considered a ‘sink’ for insulin, the consequences of reduced BMR and increased fat content are magnified with the addition of insulin-resistant diabetes. Ideally, we maintain our muscle mass throughout life as most people believe that, once we lose it, it is gone for ever.
WBV and muscle function
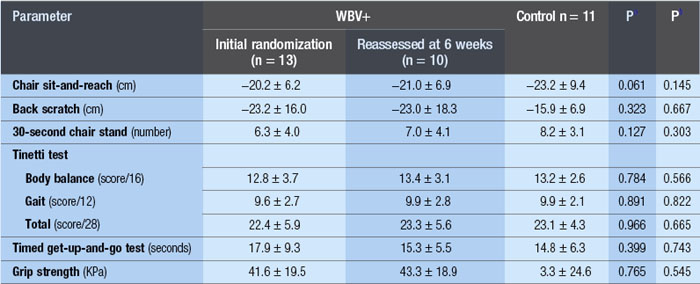
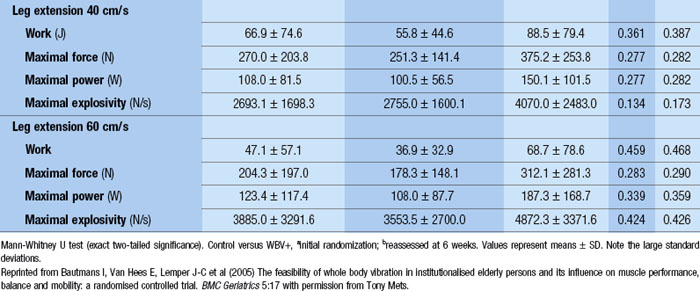
WBV has been used successfully to reduce paraspinal muscle atrophy during 8 weeks of bed rest (Belavý et al 2008). Significant improvements in fat-free mass and a 24.4% improvement in knee extension strength was demonstrated after 24 weeks of three times per week of WBV (Roelants et al 2004b). In another investigation, using a population of postmenopausal women, these authors demonstrated improved knee extensor muscle strength (16%) and improvements in counter movement jump by 2.8% after 12 weeks of WBV training (Roelants et al 2004a). Enhanced stability in movement velocity, maximum point excursion and directional control was shown with 20Hz, 3 mins/day, 3 days/week of WBV for 3 months in older women (Cheung et al 2007). The results of a 6-week training programme of WBV with 42 elderly nursing home residents were presented to the World Health Organization; seven items of the SF-36 improved, which included physical function (143%), pain (41%), vitality (60%) and general health (23%). Also, improvements in the quality of walking (57%), equilibrium (77%) and, get up and go, (GUG) 39% were shown (Bruyere et al n.d.). However, a subsequent publication by the same authors demonstrated much less significant results (Bruyere et al 2005). Moreover, similar to an investigation by Bautmans et al (2005) (see Table 3.1), the standard deviations suggest that there were large improvements in some people and not in others. If this is the case, then individual exercise prescription is critical to clinical outcome. Additionally, elderly populations have reduced heat shock protein (HSP) function suggesting that the recovery period between bouts of WBV may be critical.*
Immune function and sarcopenia
Viewing muscle as an organ of the immune system has become a rather novel and intriguing health issue. Tantalizing speculation from an anthropometrical perspective would suggest that as our forebears left the savannahs of Africa they would have required lean muscle mass for practically every aspect of life including hunting, ambulation and even thermoregulation. Additionally, lean muscle mass represented an important sink of protein which the immune system could draw upon when it encountered new pathogens in the regions where they were treading for the very first time. In contrast, modern men and women ambulate far less than previous generations, use air conditioning and central heating for thermoregulation and usually do not go hunting with a spear. Therefore, modern muscle is underutilized and could represent a danger of leaving our other organs to ‘fend for themselves’. It is plausible that WBV could represent unique myogenic stimuli which could enhance immune function through the hypertrophy of muscle tissue, activation of HSP, enhanced modulation of cytokines, improved phagocytosis and muscle-induced changes to lymphatic drainage.
WBV training was found to be as efficient as a fitness programme for increasing isometric and explosive knee extension strength and muscle mass of the upper-leg in community-dwelling older men (Bogaerts et al 2007). This is significant as even a 10% loss in lean body mass (LBM) corresponds with impaired immune function (Demling & DeSanti 1997) and a loss of approximately 30% of the body proteins can result in death (Rasmussen & Phillips 2003). During severe trauma, such as burns, the need for essential amino acids drives the catabolic loss of protein from skeletal muscle, which can be as high as 1% per day of illness (Griffiths et al 1999). Accelerated muscle proteolysis is the primary cause of this loss of LBM, which is characteristic of many diseases (Mitch & Goldberg 1996). Some peptides generated by the breakdown of cell proteins are transported to the cell surface where they are presented to cytotoxic lymphocytes, which in turn destroy cells presenting as foreign (e.g. viral) peptides (Mitch & Goldberg 1996). Consequently, successful ageing has been associated with the preservation of muscle mass (Mariani et al 1999) as this would allow these individuals to draw on their store of protein for any infectious–inflammatory–immune responses.
Increased cytokine activity has been associated with ageing and muscle weakness (Ferrucci et al 2002). This age-associated progressive dysregulation of immune response (Apovian 2000) is seen in older women with high interlukin (6) serum levels who have a higher risk of developing physical disability and experience steeper declines in walking ability than those with lower levels (Ferrucci et al 2002). However, the statistical interaction of IL-6 concentration with disability was shown to be non-linear and the progression of disability with IL-6 concentration over time was not investigated (Ferrucci et al 2002). Therefore, it is difficult to conclude whether elevated levels of IL-6 are the cause or the effect of skeletal muscle weakness (Ferrucci et al 2002; Bruunsgaard et al 2003). Furthermore, other authors suggest the IL-6 inhibits TNF-alpha production and insulin resistance (Bruunsgaard et al 2003). Nevertheless, a cycle of inactivity with a chronically impaired inflammatory–immune response is plausible. Therefore, exercise apparatus which stimulates muscle kinetic strength and hence power should inherently enhance cytokine regulation through the anabolic input of enhanced functional capacity.
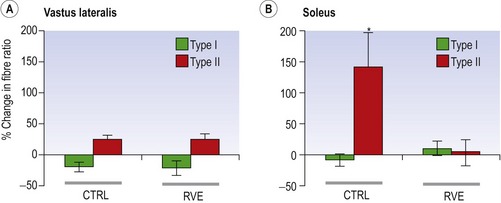
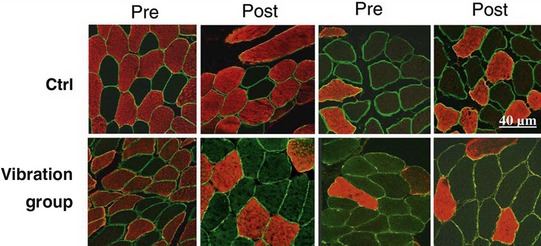
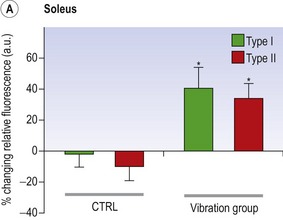
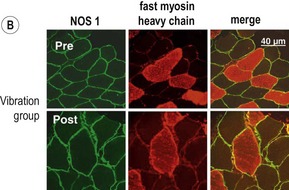
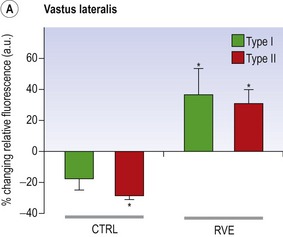
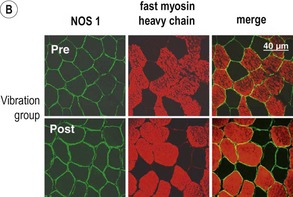
Blottner et al (2006) used 55 days of bed rest and WBV to demonstrate the preservation of muscle tissue in the soleus muscle. The disrupted pattern of myofibres, with a transformation by up to 140% from type I to type II phenotype (slow to fast twitch), did not occur in the vibration group (Fig. 3.1). Moreover, these researchers were able to demonstrate increased nitrous oxide synthase (NOS) in the soleus muscle of the WBV group (Figs. 3.2 and 3.3). They stated that this was significant as NOS1 is associated with the sarcolemmal dystrophin–glycoprotein complex via syntropin, which is linked to the subsarcolemmal actin network (Bredt 1999).
Besides cytokines, HSP have also been implicated in enhanced immune function with regular exercise. Unfortunately, numerous attempts to link exercise to meaningful alterations in immune function have been largely unconvincing (Moseley 2000). It has been argued that it is the local as opposed to the global immune system activation by HSPs which is at the core of immune effects of exercise (Moseley 2000). HSPs are involved in protein folding and sorting, in the assembly of protein complexes and in the binding of denatured proteins, and are primarily induced in response to stress (Puntschart et al 1996). Exercise may provide a hormonal stimulus to regulate HSP proteolysis. Recently, rodent investigations have demonstrated that insulin-like growth factor 1 (IGF-1) inhibits both lysosomal and ubiquitin-proteasome-dependent stress protein breakdown in skeletal muscle (Fang et al 2002), thus suggesting a hormonal regulating mechanism. Significantly, increased IGF-1 concentrations have been demonstrated near the Z bands in the elderly after a resistance training regimen (Urso et al 2001). In particular, eccentric exercises have been associated with damage to these Z bands (Fielding et al 1996). Presumably there is a role for HSP during the recovery from such damage.
In rodents, overloading of atrophied muscles, after a period of immobility, stimulates early increases in IGF-1 in slow twitch oxidative red fibres more than in fast twitch glycolytic white fibres. Release of IGF-1 was considered to be part of the myogenic reactive compensatory process of muscle hypertrophy (Adams et al 1999). IGF-1 induces muscle regulatory factor (MRF), which is facilitated by the inactivation of glycogen synthase kinase (GSK-3beta). This has the potential not only to aid in hypertrophy but also to stimulate myoblastic regrowth (van der Velden et al 2006). In contrast, HSP25 released after muscle overload was greater in the fast twitch plantaris than in the slow twitch soleus, and occurred immediately on overloading and was shown to be greatest between 3 and 7 days post stimuli. Similarly, turmour necrosis factor alpha (TNF-alpha) was increased in the fast twitch plantaris muscle but not in the slow twitch soleus 0.5–2 days after overloading. This last finding was associated with HSP25 in C2C12 myotubes, suggesting greater mechanical stress inflammatory responses in the fast twitch muscles (Huey et al 2007). Therefore, the direct correlation between IGF-1 production in muscle and the regulation of protein synthesis after HSP-induced proteolysis as a result of exercise-induced trauma is tenuous at best. Nevertheless, taken together these investigations demonstrate marked immune reactions within deconditioned muscle after exercise.
Consensus indicates that ‘moderate exercise’ may enhance immune function and may reduce the incidence of infection while long-term exhaustive exercise results in immunosuppression and an increased susceptibility to infections (Armstrong & VanHeest 2002, Dressendorfer et al 2002, Gleeson 2000, Pedersen et al 1998, Woods et al 1999). This is consistent with Ji (2002), suggesting that the major benefit of non-exhaustive exercise is to induce a mild oxidative stress that stimulates the expression of antioxidant enzymes, as well as the induction of IGF-1 (Fang et al 2002) seen in resistance training (Fiatarone Singh et al 1999, Urso et al 2001). Additionally, resistance training accompanied by nutritional supplementation has been shown to result in significant muscle hypertrophy (Fiatarone et al 1994). Biomechanical principles dictate that, for the same force, the strain in a skeletal muscle is reduced proportional to the skeletal muscle’s cross-sectional area (Hunter et al 1998). Therefore, if contractile skeletal muscle mass is maintained or enhanced, then it is plausible that a greater spectrum of ‘moderate exercise’ can be entertained.
Correctly dosed and progressed WBV probably represents moderate exercise in the vulnerable populations such as the frail elderly, those having undergone prolonged bed rest and people suffering from obesity, diabetes and/or cardiovascular disease. Inflammation and ischaemic reperfusion activates both pro- and anti-apoptotic events in cultured endothelial cells (Yang et al 2006). Ninety minutes of cycling at 2400 m altitude with a 30-Hz, 4-mm amplitude vibration significantly increased vascular endothelial growth factor (VEGF) (Suhr et al 2007). Yang et al (2006) stated that cholesterol-rich, plasma membrane rafts serve an organizational and integrational role in signal transduction which can be activated by oxidative stress. Moreover, HSP27 and HSP70 have been implicated in these inflammatory reactive oxygen species (ROS)-related processes. Rittweger et al (2001) examined oxidative stress, by using 26-Hz, 6-mm WBV to demonstrate increased oxygen consumption of 4.5 mL/min/kg. By using oxygen at 20.9 J/mL = 1.6 W (kg body mass) and walking speed at 0.4 m/s it can be calculated that this requires 2.3 mL/min/kg; therefore 3 min of standing, squatting and holding weights during WBV is metabolically comparable to walking.
Although Cubano and Lewis (2001) using in vitro space flight simulation reported that vibration stress per se had not been shown to affect HSP70, their data demonstrated that vibrated cells underwent an oxidative stress whereby glucose consumption was increased and that a decrease of approximately 30% in RNA for HSP70a/b occurred 48 hours after vibration. When prescribing moderate exercise, these findings may have important implications for the period between doses of WBV. In fact, similar to progressive resistance training (PRT), the consensus may be a stimulus every 72 hours for the treatment of conditions such as metabolic syndrome.
Importantly, WBV is dose-sensitive and can be applied for short periods of time and in varying degrees of weight-bearing using apparatuses such as the tilt table, or by simply loading the body in various positions of knee bend, one- or two-legged standing, with/without free weights. The safety of the methodology can be extrapolated from the data of Roelants et al (2004a) in which after 24 weeks of 35–40-Hz, 2.5–5.0-mm WBV, only one person dropped out due to anterior knee pain compared with six in the PRT group. Similarly, Bautmans et al (2005) demonstrated a 96% completion rate with three times per week for 6 weeks of 30–40 Hz of WBV, and Kawanabe et al (2007) reported no serious adverse events in elderly people during 12–20 Hz, 4 min, once per week over 2 months.
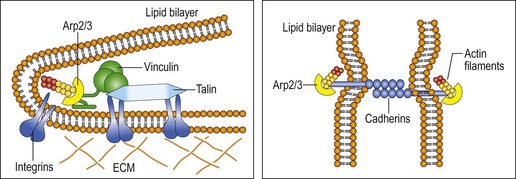
Interestingly, some of the same elements discussed in the previous chapter regarding tensegrity and WBV are also considered to be involved in protection against apoptosis. Oxidants have been shown to impair formation of focal adhesion by stimulating the activity of calpain, leading to degradation of talin and alpha-actinin. Moreover, cell migration requires the involvement of membrane protrusion and the development of new adhesions using actin polymerization and integrin engagement through transient binding of actin-related protein (Arp2/3) to vinculin (DeMali et al 2002). Together this is significant, as coupling of the membrane protrusions with cell adhesion through cell migration, using cadherin-mediated junction formation, are important elements of phagocytosis seen in the immune system when tissue needs repair (see Fig. 3.4; DeMali & Burridge 2003). Presumably, WBV has the capacity to directly stimulate these cytoskeletal elements as well as stimulate anti-oxidants such as HSP.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree