The Shoulder 
The shoulder is a complex ball-and-socket articulation, which involves synchronized motion among four joints, the glenohumeral (GH) joint, acromioclavicular (AC) joint, sternoclavicular joint, and the scapulothoracic joint. Abnormalities of one joint may secondarily affect the other joints. The structured reporting includes the GH and AC joints, since the other two joints are only partially included within the imaging field of view.
The humerus is formed from three ossification centers, which are located at the head, lesser, and greater tuberosities. These ossifications centers appear before the age of 5 years and fuse by the age of 18 to 19 years. The glenoid articular surface is concave, and its overlying cartilage is relatively thinner in the center and thicker in the periphery. The average glenoid version is 1 to 2 degrees anteversion. The hemispheric humeral head is covered by cartilage, which is thicker in the middle and thinner at the periphery and superior aspect of the humeral head. The humerus neck-shaft angle is approximately 131 degrees (120–140 degrees). Since the glenoid cup is much smaller than the humeral head, joint stability is maintained (a) by the labroligamentous complex, which acts as passive restraint and increases the glenoid surface (b) the rotator cuff (RC), which acts as a dynamic restraint.
This chapter discusses the imaging evaluation approach and describes how to fill in the structured checklist in Box 1. Conceptual details of related MR physics and imaging protocol are discussed in the chapter on MR protocol optimization.
 IMAGE EVALUATION
IMAGE EVALUATION
The stepwise interpretation approach outlined below is only a practical guide, and all shoulder structures should be evaluated in multiple planes for optimal assessment. This will also help readers perceive which structures are best depicted/evaluated on which particular plane.
1. Line up the similar plane non–fat-saturated and fat-saturated images and synchronize them for tandem evaluation.
2. Start with the coronal images in order to evaluate the AC joint and subacromial/subdeltoid (SASD) bursa abnormalities, superior humeral subluxation, low-set acromion (AC subluxation), or lateral downsloping of the acromion. From posterior to anterior, check the teres minor muscle, infraspinatus tendon (coursing obliquely upward inserting on the middle facet), junctional area of the infraspinatus and supraspinatus tendons (straightening up), and supraspinatus tendon (running horizontally inserting on the superior facet) for tendinosis, tears, and retraction. Subsequently, look at the long head of the biceps tendon (LHBT), biceps–labral anchor, and superior and inferior labrum. Next, evaluate the (anterior and posterior bands of the) inferior glenohumeral ligament (IGL), GH cartilage, and especially the superior humeral head and the subchondral bone for edema, cystic changes, and osteophytes. Coronal images are also suitable for discriminating and assessing the (conoid and trapezoid) bands of the coracoclavicular (CC) ligament in suspected AC joint sprains. Finally, look at the RC muscles for fatty replacement, edema-like signal, and atrophy.
3. On sagittal images, assess the acromion curvature and potential anterior downsloping. Correlate the tendon abnormalities on coronal images with sagittal images to estimate the anteroposterior extent (width) of tendon tears and distinguish among the involved tendons by using cross-referencing localizers. Note that about 15 mm proximal to the insertion on the greater tuberosity, the supraspinatus and infraspinatus tendons overlap, merge, and become inseparable. Sagittal images also provide adequate evaluation of the subscapularis tendon and the horizontal (intra-articular) portion of LHBT for tendinosis and integrity. Correlation of labral abnormalities with other planes is also possible although sagittal images are not considered primary for labral evaluation. The RC muscles can also be evaluated, along with occupancy ratios in the supraspinatus and infraspinatus fossae, though it should be remembered that in cases of full-thickness tendon tears with retraction, the latter fossae will appear empty, despite the muscle bulk being normal. Therefore, it is imperative to evaluate the RC muscles on the coronal view. Finally, this plane is ideal for the assessment of the subcoracoid fat, rotator interval, and coracohumeral ligament (CHL).
4. Axial (short axis) images are essential for the evaluation of the subscapularis tendon, LHBT, superior glenohumeral ligament (SGL), middle glenohumeral ligament (MGL), anterior and posterior labrum, and the detection of (anterior or posterior) humeral decentring/subluxation. They are also useful for the detection of os acromiale and tears of the anterior-most supraspinatus tendon. The axial plane is the most useful for the evaluation of central shoulder cartilage, glenoid retroversion, glenoid bone stock for potential shoulder replacement cases, glenoid remodeling in overhead throwers (posterosuperior and superior), and glenoid dysplasia (posteroinferior and posterior portions). Other incidental abnormalities which may also be detected include lung lesions, axillary lymph nodes, and scapulothoracic bursitis.
 BOX 1: The Structured Report: Shoulder
BOX 1: The Structured Report: Shoulder
The checklist for structured reporting of MR imaging of the shoulder. For each field, Normal is considered default in the dictation, whereas the rest of the elements describe various pathologies that could be encountered during imaging evaluation. See Appendices 1 and 2 at the end of the chapter for sample completed reports for normal and abnormal exam results.
EXAM: MRI OF SHOULDER
FINDINGS:
Alignment: [<Normal> <Anterior / Posterior / Superior glenohumeral subluxation>]
Fluid:
Subacromial/subdeltoid bursa: [<Normal> <Mild bursitis> <Moderate or large bursitis> <Partial bursal rupture>]
Glenohumeral: [<Normal> <Small effusion> <Moderate effusion> <Large effusion> <Cartilaginous or osteochondral bodies> <Synovial hypertrophy>]
Long head of biceps brachii tendon: [<Normal> <Small fluid> <Tenosynovitis> <Cartilaginous or osteochondral bodies>]
Acromial arch:
Shape: [<Flat / Curved / Hooked / Convex> <Low-set acromion>]
Subacromial spur: [<Absent / Present> <Keel / Heel / Traction / Bird beak spur>]
Lateral / anterior downsloping: [<Absent / Present>]
Acromioclavicular joint: [<Normal> <Osteoarthrosis> <Sprain>]
Rotator cuff:
Supraspinatus: [<Normal> <Mild / Moderate / Severe tendinosis> <Bursal / Articular-sided fraying> <Tear> <Retraction> <Muscle atrophy>]
Infraspinatus: [<Normal> <Mild / Moderate / Severe tendinosis> <Bursal / Articular-sided fraying> <Tear> <Retraction> <Muscle atrophy>]
Subscapularis: [<Normal> <Mild / Moderate / Severe tendinosis> <Tear> <Retraction> <Muscle atrophy>]
Rotator interval and long head of biceps brachii tendon:
Rotator interval: [<Normal> <Synovial thickening> <Acute sprain>]
Biceps–labral anchor: [<Intact> <Tear>]
Horizontal portion: [<Normal> <Mild / Moderate / Severe tendinosis> <Tear>]
Vertical portion: [<Normal> <tendinosis> <Tear>]
Genu: [<Normal> <Mild / Moderate / Severe tendinosis> <Tear> <Subluxed>]
Glenohumeral joint:
Labrum: [<Normal> <Degenerative fraying> <Tear> <Paralabral cyst>]
Glenohumeral ligaments: [<Normal> <Thickening / Acute sprain of ligament>]
Glenohumeral cartilage: [<Normal>]
Bones: [<Normal> <Greater tuberosity / Lesser tuberosity cysts> <Enthesopathy>]
Muscles: [<Otherwise normal>]
Vessels: [<Normal>]
Nerves: [<Normal>]
Other:
IMPRESSION:
[<In the order of importance with acute findings first>]
 HOW TO FILL THE STRUCTURED REPORT
HOW TO FILL THE STRUCTURED REPORT
ALIGNMENT: [<Normal> <Anterior / Posterior / Superior glenohumeral subluxation>]
The evaluation of bony alignment is best performed on non–fat-suppressed images. Normal alignment means bony articulation congruency at the AC and GH joints, as well as an acromiohumeral distance of greater than 9 mm. In RC pathology (tendinosis/tears), there is often posterior, superior, or posterosuperior decentering (aka ascent or subluxation) of the humerus with narrowing (<8 mm) of the acromiohumeral distance (Fig. 1). Less commonly, there is anterior or inferior humeral subluxation. Instability is clinically tested using anterior or posterior apprehension tests for anterior and posterior instability, respectively. Constituted by the acromion posteriorly, and the coracoid process and coracoacromial ligament anteriorly, the coracoacromial arch contains the SASD bursa, supraspinatus muscle and tendon, and LHBT. It is best evaluated on coronal and sagittal images. The coracoacromial ligament has two bundles (anterolateral and posteromedial) and prevents anterosuperior translation of the humeral head in the setting of rotator interval and/or RC injuries. At the AC joint, a low-set (inferiorly subluxed) acromion may be present, indicating prior injuries to the AC capsule, inferior, and/or superior AC ligaments (Fig. 2). Thickening, attenuation, or deficiency of one or both of these ligaments should be reported. A common pattern observed in weight lifters and overhead throwers includes low-set acromion, deficient or attenuated inferior AC ligament and thickened superior AC ligament.
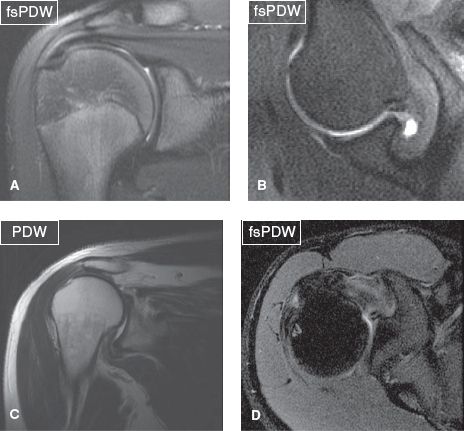
Fig. 1: Normal and abnormal alignment. Coronal image (A) and axial image in abduction and external rotation (ABER) (B) demonstrate normal anatomic relationship between the humeral head and the glenoid. Coronal (C) and axial (D) images show superior and posterior humeral subluxation, respectively.
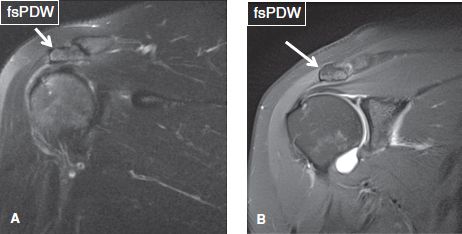
Fig. 2: Low-set acromion. On coronal images (A, B), the acromion (arrows) is parallel to but lower in position with respect to the clavicle. Notice the thickened superior AC ligament in (B) and attenuated inferior AC ligament in both images.
FLUID:
Subacromial/subdeltoid bursa: [<Normal> <Mild bursitis> <Moderate or Large bursitis> <Partial bursal rupture>]
Glenohumeral joint: [<Normal> <Small effusion> <Moderate effusion> <Large effusion> <Cartilaginous or osteochondral bodies> <Synovial hypertrophy>]
Long head of biceps brachii tendon: [<Normal> <Small fluid> <Tenosynovitis> <Cartilaginous or osteochondral bodies>]
The normal SASD bursa lies under the acromion and deltoid muscle, extends up to the level of the humeral metaphysis level, is normally less than 2 mm thick, and shows minimal T2 hyperintense signal. Any fluid layer equal or greater than 2 mm in thickness indicates mild bursal distention, which could be related to full-thickness RC tear, partial bursal-sided tear, injury, or inflammatory/infectious bursitis. Although there are no defined rules, mild bursitis refers to fluid reaching laterally to the level of the acromion, mild to moderate bursitis refers to fluid underneath the acromion and deltoid muscle belly, whereas moderate refers to significant distention of the bursa at both places (Fig. 3). Severe bursal distention is uncommon and is usually due to long-standing inflammatory conditions, such as rheumatoid arthritis or chronic massive RC tears. In addition, one should look for internal synovial thickening (indicating chronic bursitis); adjacent fascial, muscle, or bone marrow edema (may indicate infection); calcific hypointense debris (calcium hydroxyapatite deposition); rice bodies (rheumatoid arthritis); and synovial chondromatosis (uniform in size and shape, numerous rounded bodies) (Figs. 4, 5). Finally, fascial fluid extending below the humeral metaphysis and medially under the acromion or around the RC muscles indicates recent partial bursal rupture and, in most cases, is related to recent trauma (fall) (Fig. 6). In subacute/chronic stages, these leaks can seal and form a synovial diverticulum (presenting as a unilocular fluid collection with a neck) or a ganglion cyst (presenting as a multilocular fluid collection with/without a definite neck).
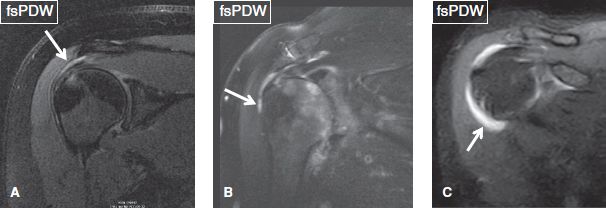
Fig. 3: Fluid within the subacromial/subdeltoid bursa (arrows). Coronal images. A: Small amount of fluid is evident within the bursa, in keeping with mild bursitis. B: The bursa contains fluid, which extends underneath the deltoid muscle, in keeping with mild to moderate bursitis. C: There is substantial fluid distention of the bursa, with fluid tracking underneath the deltoid muscle. This case is compatible with moderate bursitis.
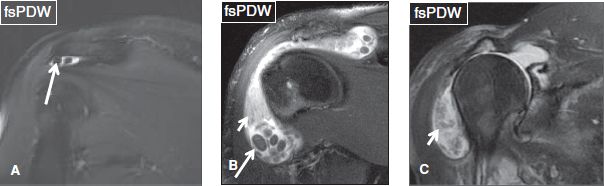
Fig. 4: Coronal images (A–C) demonstrate multiple bodies (long arrows) within the subacromial/subdeltoid bursa. In (B) and (C), large fluid distention and synovial thickening (short arrows) indicate a chronic inflammatory process.
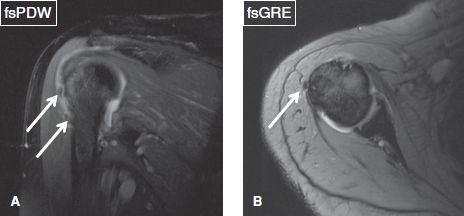
Fig. 5: Calcific subacromial/subdeltoid bursitis. Coronal (A) and axial (B) images demonstrate hypointense ovoid bodies (arrows) within the subacromial/subdeltoid bursa, which correspond to calcium hydroxyapatite deposition.
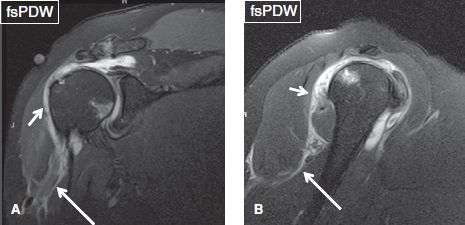
Fig. 6: Coronal (A) and sagittal (B) images demonstrate moderate fluid collection in the subacromial/subdeltoid bursa (short arrows), as well as an adjacent ill-defined fluid tracking below the humeral metaphysis (long arrows) and around the subscapularis muscle, indicating bursal rupture.
The GH joint normally contains minimal fluid, which does not significantly distend the capsule. It includes an anterosuperior subscapularis recess, an inferior (axillary) recess, and a posterior recess. Synovial thickening and loose bodies commonly develop and lodge in these recesses, respectively. The joint capsule communicates with the LHBT sheath and occasionally features a superior plica (Fig. 7). In cases of adhesive capsulitis (joint contraction), fluid normally and preferentially collects in the subscapularis recess and the LHBT sheath. Therefore, one should not overdiagnose biceps tenosynovitis in such cases. In small effusion, there is mild distention of the joint, predominantly involving the inferior dependent recess, whereas in moderate effusion there is distention of all recesses (Fig. 8). Similar to the SASD bursa, large distention occurs in inflammatory conditions such as rheumatoid arthritis, pigmented villonodular synovitis (PVNS), synovial osteochondromatosis, septic arthritis, or significant trauma. One should look for and report synovial thickening (indicating long-standing internal derangement), loose bodies, and/or blood clots (Figs. 8, 9). Hemarthrosis, depicted as T1 hyperintense joint effusion, may be related to recent trauma (look for associated local bony or soft tissue injury), hemophilia (look for associated enlargement of the humeral head and fluid–fluid levels), or vascular malformation. Finally, capsular injuries with fascial fluid (from trauma, humeral subluxation, or dislocation), synovial diverticulae, and ganglion may also be identified. Synovial diverticulae are particularly common following prior arthroscopy or surgical procedures, probably formed from pseudo-encapsulation of capsular leaks caused by fluid distention, which is required during these procedures. Best depicted on sagittal images, the subcoracoid bursa is a separate bursa, which is located anterior to the subscapularis muscle, and extends along the entire superoinferior extent of the latter, as opposed to the subscapularis recess, which occupies only the upper third of the subscapularis. The subcoracoid recess communicates with the SASD bursa; therefore, its distention can be used as indirect sign of full-thickness RC tear (Figs. 10–12).
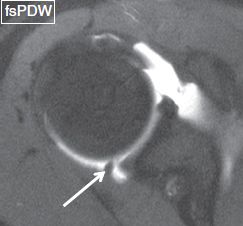
Fig. 7: Superior plica. Axial image reveals a thick plica (arrow) in the posterosuperior portion of the joint.
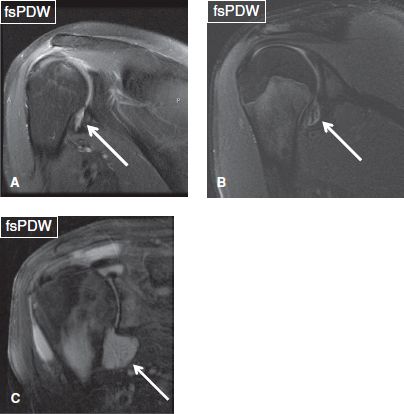
Fig. 8: Glenohumeral joint effusion. Coronal images show small (A, B) and moderate (C) glenohumeral joint effusions (arrows). In (B), loose bodies are evident within the joint space.
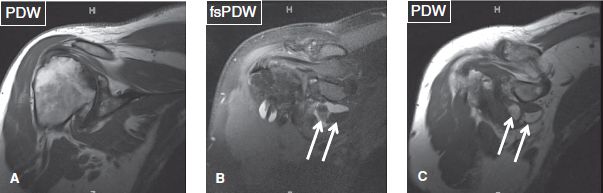
Fig. 9: Synovial osteochondromatosis of the glenohumeral joint. Coronal images (A–C) displaysevere osteoarthritis of the glenohumeral joint. In (B) and (C), ossified intra-articular loose bodies (arrows) reflect secondary osteochondromatosis.
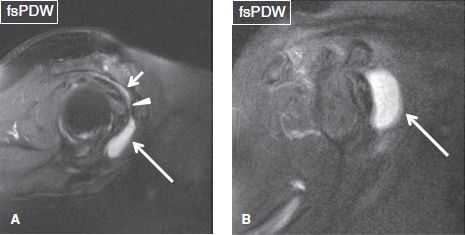
Fig. 10: A: Sagittal image exhibits mild subacromial/subdeltoid bursitis (short arrow) and concomitant moderate subcoracoid bursitis (long arrow). Arrowhead shows the communication between the two bursae. B: Sagittal image from another subject with moderate subcoracoid bursitis (arrow). Both subjects had full-thickness cuff tears (not shown).
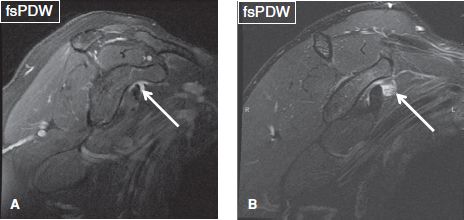
Fig. 11: Coronal images (A, B) demonstrate small fluid collection within the subscapularis recess (arrows). In (B), heterogeneous fluid content is due to synovial chondromatosis.
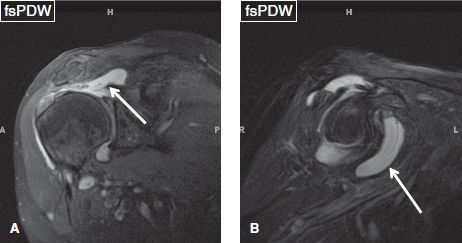
Fig. 12: Subcoracoid bursa distention in the setting of full-thickness rotator cuff tear. A: Coronal image demonstrates full-thickness tears of the supraspinatus tendon, with proximal retraction (arrow). B: Corresponding sagittal image exhibits fluid distention of the subcoracoid bursa (arrow).
Because of its normal communication with the shoulder joint, the LHBT sheath normally contains trace amount of fluid. Tenosynovitis is established only if the fluid is circumferential around the tendon and if it is disproportionate with respect to the shoulder joint fluid. Circumferential fluid around the LHBT may also be seen in the setting of significant GH joint effusion (due to the aforementioned communication) or adhesive capsulitis (due to preferential pooling of fluid, related to thickened and contracted capsule). In the latter cases, coexisting tenosynovitis cannot be excluded, but the possibilities become higher if there is LHBT sheath synovial thickening, LHBT tear, or tendinosis. Similar to other synovial spaces, synovial leaks can occur with acute trauma (usually weight lifting injuries), whereas in subacute/chronic stages, diverticula and ganglion can arise from the tendon sheath (Figs. 13–15). It is important not to confuse in-plane flow phenomenon in the circumflex humeral vein (branching structure) with synovial diverticulum of the biceps tendon sheath.
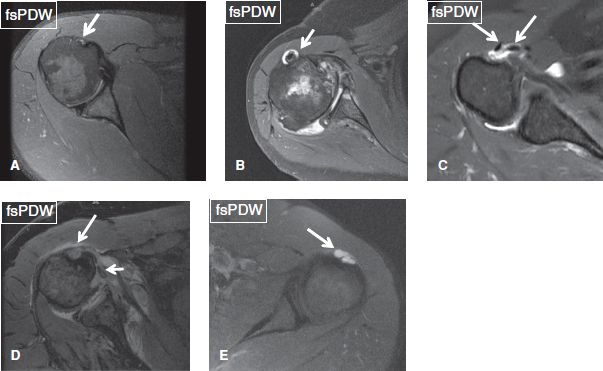
Fig. 13: Normal and abnormal long head of the biceps brachii tendon. Axial images. A: Normal long head of the biceps brachii tendon (arrow), located within the bicipital sulcus and surrounded by trace amount of fluid. B: Small amount of fluid (arrow) surrounds the tendon with synovial thickening, in the setting of shoulder effusion. C: Moderate amount of fluid surrounds the tendon, which is congenitally bifid (arrows). D: Rupture of the tendon sheath with fluid extravasation (long arrow) and medial tendon dislocation (short arrow). E: Synovial diverticulum of the tendon sheath (arrow).
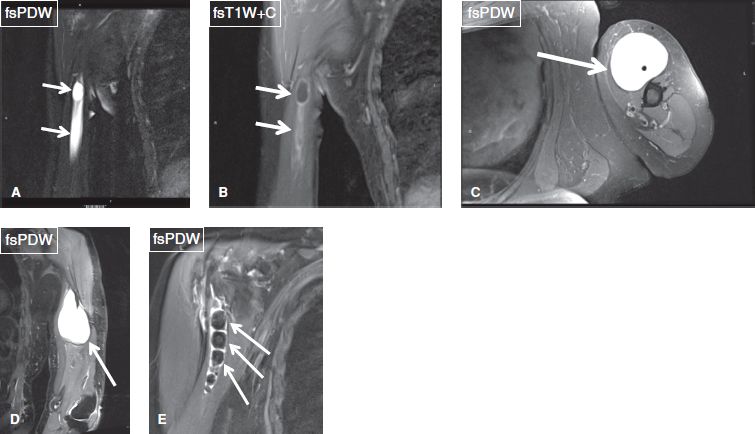
Fig. 14: Abnormalities of the long head of the biceps brachii tendon sheath. A, B: Coronal images exhibit ganglion from the biceps tendon sheath (arrows). Notice peripheral enhancement on contrast in (B), which excludes the possibility of tumor. C, D: Axial (C) and coronal (D) images show a ganglion cyst (arrows) of the biceps tendon sheath. E: Multiple hypointense loose bodies (arrows) are evident within the biceps tendon sheath, in this case of synovial chondromatosis of the long head of the biceps tendon sheath.
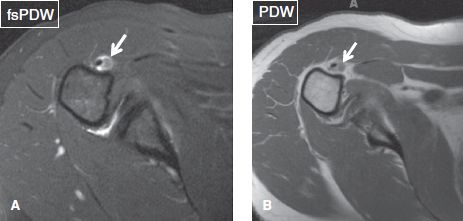
Fig. 15: Clot within the biceps tendon sheath. Axial images (A, B) show an isointense ill-defined lesion within the biceps tendon sheath, corresponding to a blood clot (arrows).
ACROMIAL ARCH:
Shape: [<Flat / Curved / Hooked / Convex> <Low-set acromion>]
Subacromial spur: [<Absent / Present> <Keel / Heel / Traction / Bird beak spur>]
Lateral / Anterior downsloping: <Absent / Present>]
Acromioclavicular joint: [<Normal> <Osteoarthrosis> <Sprain>]
The AC joint is formed by the distal end of the clavicle and the acromion and normally contains trace amount of synovial fluid and a hypointense articular disc. Thickenings of the joint capsule form the superior, posterior, and inferior AC ligaments, which provide anteroposterior static joint stability. Superoinferior static joint stability is provided by the CC ligament, which has two bands, the (triangular-shaped) posteromedial conoid band and the (quadrilateral-shaped) anterolateral trapezoid band. Functionally, the conoid band is the more important. The deltoid and trapezius muscles provide further dynamic reinforcement to the AC capsule and ligaments.
The acromial shape is best assessed on sagittal images, midway between the AC joint and the outer border of the acromion. If evaluated too close to the joint, a curved acromion may look like hooked. The most common acromial shape is flat (type I) or concave (type II), whereas convex or hooked (type III) acromion shapes are rare (Fig. 16). The acromion normally parallels the curvature of the humeral head. Lateral and anterior downsloping is detected on coronal and sagittal non–fat-suppressed proton density-weighted (PD) images, respectively, indicated by lack of parallel orientation between the acromion and the humeral head (Fig. 17).
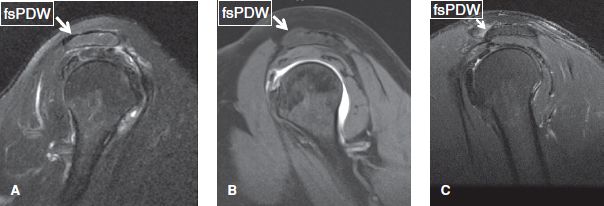
Fig. 16: Sagittal images demonstrate flat (A), concave (B), and hooked (C) acromion (arrows).
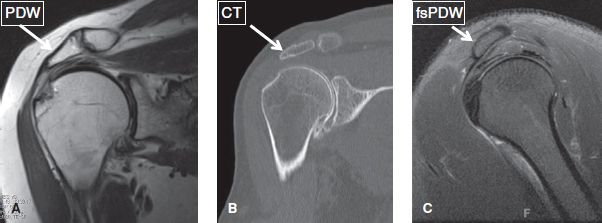
Fig. 17: Acromial downsloping. MR (A) and CT (B) images in the coronal plane show a laterally downsloping acromion (arrows). C: Sagittal MR image displays an anteriorly downsloping acromion (arrow).
Painful compression of the supraspinatus tendon and subacromial bursa between the coracoacromial arch and the humeral head is referred to as subacromial impingement (anterosuperior or extrinsic impingement). However, impingement is a clinical diagnosis, and one can only comment on whether anatomy associated with impingement is present (primary extrinsic impingement) or absent (secondary extrinsic impingement and internal impingement). Secondary extrinsic impingement may be related to myotendinous fatigue or capsular laxity, the latter, particularly resulting in multidirectional instability. Internal impingement is related to capsular stiffening posteriorly and inferiorly with laxity anteriorly, commonly seen in overhead throwers, as detailed below.
It should be noted that the more important findings related to subacromial impingement (primary extrinsic impingement) are the presence or absence of a subacromial spur and downsloping acromion rather than the acromion shape itself. Subacromial spurs are best seen on coronal non–fat-suppressed PD images, since hypointense coracoacromial ligament thickening or deltoid muscle origin from the acromion can mimic a spur on fat-suppressed PD images. There are five types of spurs that include traction spurs (enthesophytes at the deltoid attachment), bird beak spurs (traction spurs plus remodeled concave undersurface acromion due to superiorly riding humerus), keel spurs (enthesophytes at the coracoacromial ligament attachment), heel spurs (combination of traction, undersurface remodeling, and keel components), and medial spurs (part of AC osteoarthritis [OA]) (Figs. 18, 19). Traction spurs are usually associated with RC tendinosis/partial tears. Bird beak and keel spurs are commonly associated with partial RC tears, whereas heel spurs are usually associated with full-thickness RC tears. Impingement is clinically tested by using the Neer test (arm in full flexion) or the Hawkin test (arm in 90 degrees forward flexion and internal rotation). The Neer test is performed by placing the arm in forced flexion and full pronation. The scapula should be stabilized during the maneuver to prevent scapulothoracic motion. Hawkin test is particularly directed to supraspinatus evaluation and is considered to be more sensitive than the positive Neer sign. Notice that RC pathology manifests with weakness more than pain versus other pathologies, such as SASD bursitis and AC joint arthritis, which manifest with pain more than weakness.
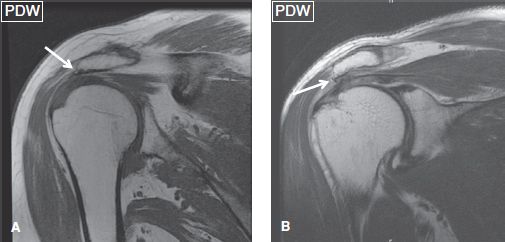
Fig. 18: Acromial spurs. Coronal images show traction (arrow in A) and bird-beak (arrow in B) acromial spurs.
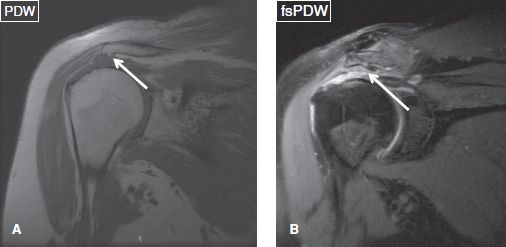
Fig. 19: Acromial spurs. Coronal images show keel (arrow in A) and heel (arrow in B) acromial spurs. Notice full-thickness rotator cuff tear in (B).
One should look for os acromiale (~3.5% prevalence in the general population), which can be bilateral in about 60% of cases. It predisposes to RC pathology, as the deltoid muscle inserts on the accessory ossicle rather than the acromion itself, and it can contribute to instability. Since the accessory ossification center for the acromion generally fuses around the age of 25 years, the diagnosis of os acromiale should be avoided in subjects younger than this age. However, the unfused ossification center can develop stress-related bone marrow edema, cystic changes, or disruption of the synchondrosis (depicted as widening of the joint space along with fluid signal), particularly in overactive athletes, such as gymnasts, earlier than the fusion age of 25 years. These findings should be reported as they can be symptomatic and predispose to frank disruption of the synchondrosis. There are four common types of os acromiale (pre-acromion—a small ossicle at the coracoacromial ligament attachment, meso-acromion—a equilateral triangular shaped larger ossicle, meta-acromion—further larger triangular ossicle, and basiacromion—adjacent to the base of the acromion process, with the mesoacromion being the most common). Os acromiale is best identified on axial images, whereas the above-described pathologic stress related imaging findings are equally well seen on axial and coronal images (Fig. 20). Similar to AC joint pathology although less commonly, one may encounter fluid distention of the os acromiale synchondrosis, stress-related bone marrow edema across the synchondrosis, synovial diverticula(e), or even geyser phenomenon (fluid outpouching in the subcutaneous tissues from completely/partially disrupted synchondrosis) (Figs. 21–23).
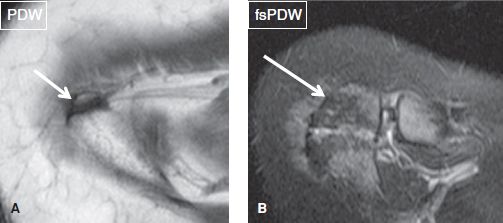
Fig. 20: Types of os acromiale. Axial images exhibit a preacromion (arrow in A) and a mesoacromion (arrow in B).
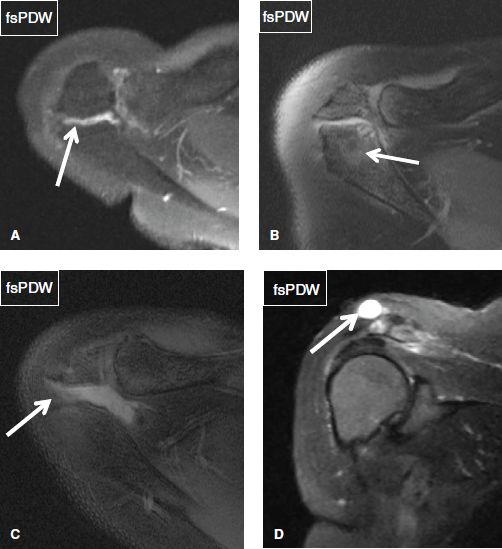
Fig. 21: Os acromiale abnormalities. A: Axial image shows trace fluid collection (arrow) within the os acromiale synchondrosis. B: Axial image demonstrates stress-induced bone marrow edema (arrow) across the os acromiale synchondrosis, along with subarticular cystic changes. C: Axial image exhibits disruption and moderate fluid distention (arrow) of the os acromiale synchondrosis. D: Coronal image shows a well-defined fluid collection (arrow) superior to a partially disrupted os acromiale synchondrosis, corresponding to geyser phenomenon.
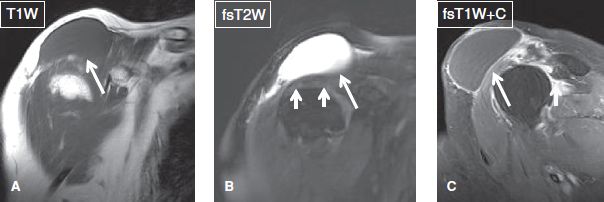
Fig. 22: Geyser phenomenon. Coronal conventional (A, B) and sagittal contrast-enhanced (C) images show a well-defined fluid collection (long arrows) adjacent to the acromioclavicular joint. The cyst is believed to result from leakage of synovial fluid through the joint due to a massive rotator cuff tear (short arrows in B). Note associated proximal retraction of the supraspinatus tendon (short arrow in C).
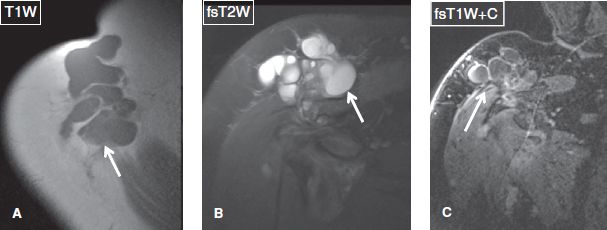
Fig. 23: Geyser phenomenon mimicking sarcoma. Axial (A) and coronal (B) unenhanced images and coronal contrast-enhanced image (C) show a subcutaneous multiloculated and lobulated fluid collection projecting superior to the shoulder joint. Without image (C), which confirms the absence of solid components, the possibility of a soft tissue sarcoma cannot be entirely excluded.
AC joint OA should be classified as mild, moderate, or severe. Associated findings, such as capsular thickening, bony hypertrophy, subchondral edema, sclerosis, and cystic changes should be reported. OA grading is similar to other joints, with capsular thickening/small osteophytes suggesting mild OA, mild–moderate cartilage loss with subchondral cysts/sclerosis/significant edema with moderate OA, and large osteophytes, bony deformities and greater than 50% cartilage loss with severe OA (Fig. 24). AC joint pathology is clinically tested using the “cross arm test,” in which the arm is put into forward elevation to 90 degrees with active adduction. AC joint OA should be differentiated from AC joint sprains, which usually affect younger individuals and those involved in bench pressing. AC joint sprains are associated with capsular thickening, superior and inferior AC ligament thickening/attenuation/tears, and feature the key finding of periarticular fascial edema. However, underlying OA may also be present, especially in older individuals. Other associated findings may include AC joint widening with or without post-traumatic osteolysis (erosions/edema of the joint surfaces), CC ligament sprain, and deltoid or trapezius muscle strains apart from history of recent trauma. AC joint injuries can be graded according to the Rockwood classification (Table 1) (Figs. 25–27) Types I to III are typically treated conservatively, whereas types IV to VI usually require surgery. There is increased risk of infection in type IV injury. One should also look for related differential diagnoses or associated injuries, such as distal clavicle fracture (isolated clavicle-sided bone marrow and fascial edema, along with a hypointense subchondral fracture line) or posttraumatic osteolysis (ill-defined cortical margin/erosions, commonly associated with muscular body habitus) (Figs. 28, 29).
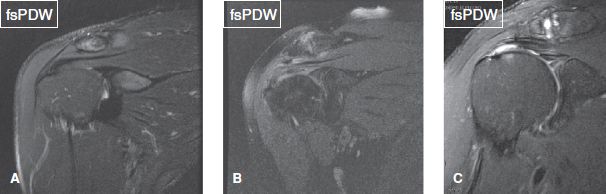
Fig. 24: Acromioclavicular joint osteoarthritis. Coronal images demonstrate a normal joint (A), and cases of mild (B), and moderate to severe (C) osteoarthritis.
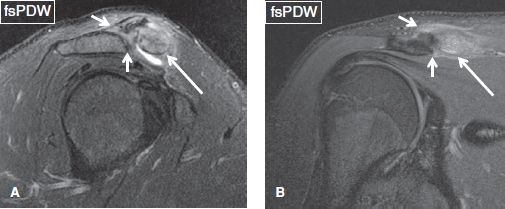
Fig. 25: Acromioclavicular joint type I sprain in the setting of clavicular fracture. On sagittal (A) and coronal (B) images, there are high-grade partial tears of acromioclavicular ligaments (short arrows) without joint space widening. Marrow edema of the distal clavicle (long arrows) is due to subchondral fracture. Notice associated fascial edema, common finding in AC sprains, unlike simple OA.
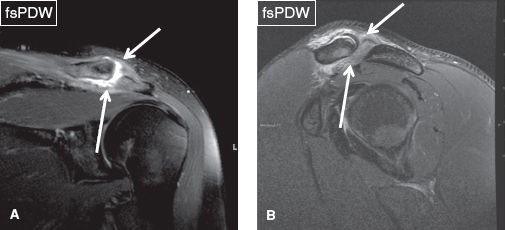
Fig. 26: Acromioclavicular joint type II sprain. Coronal (A) and sagittal (B) images demonstrate complete rupture of the superior and inferior acromioclavicular ligaments (arrows).
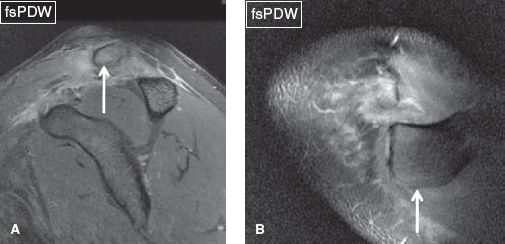
Fig. 27: Acromioclavicular joint type IV sprain. Sagittal (A) and axial (B) images demonstrate acromioclavicular joint separation along with posterior clavicular subluxation (arrows).
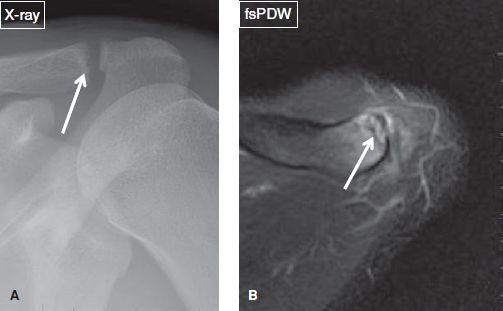
Fig. 28: Subchondral clavicular fracture. A: Radiograph displays ill-defined distal clavicular cortex (arrow). B: The corresponding axial MR image shows a subarticular hypointense fracture line (arrow) in the distal clavicle with associated bone marrow edema.
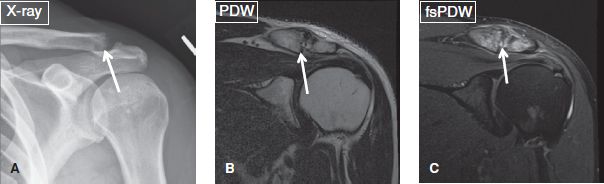
Fig. 29: Posttraumatic clavicular osteolysis. Anteroposterior radiograph (A) and coronal MR images (B, C) demonstrate cortical erosions of the distal clavicle (arrows). In (C), bone marrow edema and fascial edema are evident across the joint line.
TABLE 1: The Rockwood classification for grading acromioclavicular joint injury
ROTATOR CUFF:
Supraspinatus: [<Normal> <Mild / Moderate / Severe tendinosis> <Bursal / Articular-sided fraying> <Tear><Retraction> <Muscle atrophy>]
Infraspinatus: [<Normal> <Mild / Moderate / Severe tendinosis> <Bursal / Articular-sided fraying> <Tear> <Retraction> <Muscle atrophy>]
Subscapularis: [<Normal> <Mild / Moderate / Severe tendinosis> <Tear> <Retraction> <Muscle atrophy>]
To understand RC tears, it is important to learn the anatomic architecture in three planes. In the coronal plane, horizontally from lateral to medial, the cuff is divided into the following:
 The attachment site (leading edge/extra-articular portion, about 1 cm in width)
The attachment site (leading edge/extra-articular portion, about 1 cm in width)
 The critical zone (extending 1 cm proximal to greater/lesser tuberosity and about 1 cm in width, also known as avascular zone although a misnomer, since it can be hypervascular instead)
The critical zone (extending 1 cm proximal to greater/lesser tuberosity and about 1 cm in width, also known as avascular zone although a misnomer, since it can be hypervascular instead)
 The myotendinous junction (about 1 to 2 cm in width, usually located between 11 and 1 o’clock location)
The myotendinous junction (about 1 to 2 cm in width, usually located between 11 and 1 o’clock location)
 The muscle fibers
The muscle fibers
In the sagittal plane, the cuff is composed of the following (Figs. 30–33):
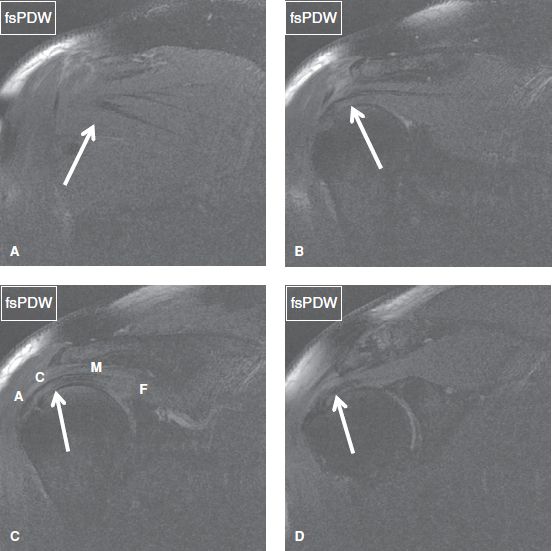
Fig. 30: The rotator cuff muscle/tendons. In posterior to anterior (A–D) coronal images, arrows denote infraspinatus (A), anterior infraspinatus and posterior supraspinatus (B), posterior supraspinatus (C), and anterior supraspinatus (D). In (C), (A) corresponds to the attachment site, (C) to the critical zone, (M) to the myotendinous junction, and (F) to the muscle fibers.
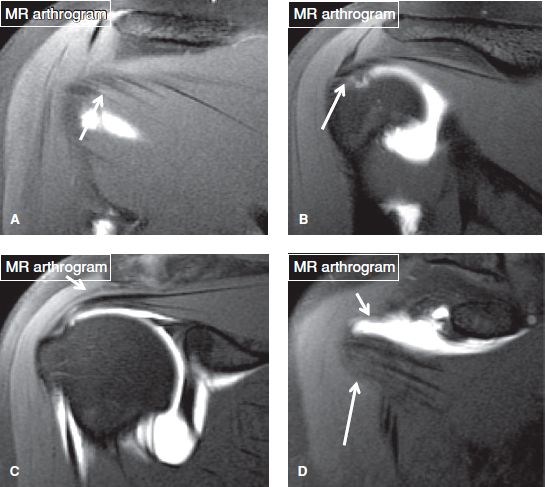
Fig. 31: The rotator cuff muscle/tendons. Long arrows in posterior to anterior (A–D) coronal MR arthrography images indicate the infraspinatus (A, B), the supraspinatus (C), and the subscapularis (D). Notice the normal gap of the rotator interval (short arrow in D).
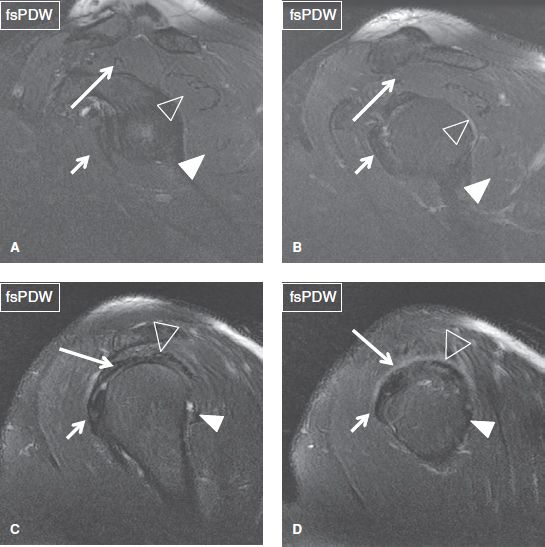
Fig. 32: The rotator cuff muscle/tendons. Arrows in medial to lateral (A–D) sagittal images indicate the subscapularis (short arrows), supraspinatus (long arrows), infraspinatus (open arrowheads), and teres major (solid arrowheads).
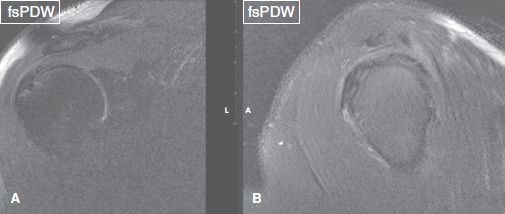
Fig. 33: The supraspinatus–infraspinatus tendon junction. Coronal image (A) corresponds to the vertical plane of sagittal image (B) and designates the junction between the supraspinatus and infraspinatus tendons, which unite into a single tendon close to the rotator cuff attachment site.
 The subscapularis anteriorly (1.8 to 2 cm in craniocaudal diameter)
The subscapularis anteriorly (1.8 to 2 cm in craniocaudal diameter)
 The supraspinatus anterosuperiorly (2 to 2.5 cm in anteroposterior width)
The supraspinatus anterosuperiorly (2 to 2.5 cm in anteroposterior width)
 The infraspinatus posterosuperiorly (1.8 to 2 cm in anteroposterior width)
The infraspinatus posterosuperiorly (1.8 to 2 cm in anteroposterior width)
 The teres minor posteriorly (a few millimeters to 1 cm in width)
The teres minor posteriorly (a few millimeters to 1 cm in width)
In the coronal plane, the RC measures 6 to 7 mm in (craniocaudal) thickness, especially in the cable area (described below). It has five layers, which are formed from superior to inferior by the CC ligament, compact tendon fibers, loosely organized tendon fibers, CHL with connective tissue fibers, and the joint capsule. Neurovascular supply to the RC derives mostly from the bursal rather than the articular side of the tendons.
Scuffing and fraying is the terminology popular among orthopods for minor cuff alterations. Scuffing refers to capsular abrasions (only seen arthroscopically), whereas fraying refers to tears of the enveloping CHL and fibrillations of the tendon fibers (can be easily seen with current high-field MR imaging techniques). RC degenerative fraying and tears should be further described as articular-sided or bursal-sided. The authors uncommonly use the term “intrasubstance tears in isolation,” since pure intrasubstance tears are less common. These lesions can be seen in the (bipennate) infraspinatus tendon more commonly compared to the (multipennate) supraspinatus and subscapularis tendon, which have intertwined myotendinous fibers. Intrasubstance extension of articular- or bursal-sided tears is much more common leading to intermediate- or high-grade tears. Such tears are frequently associated with intrasubstance fluid dissection, resulting in delaminating lesions and intramuscular cyst formation (Figs. 34, 35). Because of the laminar architecture of the tendons, fluid tends to dissect between tight and loose tendon layers. Since the teres minor tendon is seldom injured at the attachment site, the evaluation of the RC is usually limited in clinical practice to the assessment of the supraspinatus, infraspinatus, and subscapularis tendons.
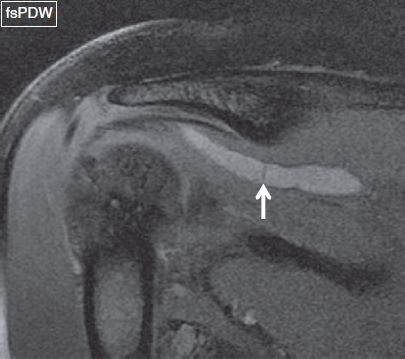
Fig. 34: Intramuscular cyst. Coronal image shows a cystic lesion (arrow) within the supraspinatus tendon, which has resulted due to seepage of fluid through the delaminating tendon tear extending from the attachment (not shown) to the myotendinous junction (arrow).
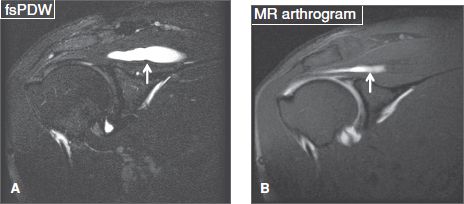
Fig. 35: Intramuscular cyst. Coronal conventional fsT2 W (A) and arthrography fsT1 W (B) images show a cystic lesion (arrows) within the supraspinatus tendon. On the latter image, the cyst partially fills with contrast.
Tendinosis and tears should both be described. However, it is important to first understand the mechanisms of RC tendinosis and tears. The initial theory of subacromial impingement, described by Neer et al., suggested that acromial hook, downsloping acromion, os acromiale, AC joint OA, thick coracoacromial ligament, and subacromial spurs are the factors which, in isolation or combination, contribute to RC impingement and/or tears. However, that theory was unable to explain why articular-sided (undersurface) RC tears occur more frequently as compared to the bursal-sided tears, if impingement was the only predisposing factor. The “fatigue failure” theory, explains the rotator cuff pathology in a more intuitive way. As a result of repetitive trauma and microinjuries caused by everyday activities or excessive stress on shoulder, recurrent RC microtears occur, particularly at its myotendinous junction. In children and relatively younger adults, the healing capacity is much more than the tearing predisposition. With increasing age, the healing capacity lags, leading to the development of RC tendinosis and focal partial tears. Tendinosis and partial tears are common in young and middle-aged individuals, whereas high-grade partial tears and tears that progress to full-thickness tears are common in the older population. This phenomenon may be further complicated by factors such as increasing stresses from recurrent overhead activities, such as in high-performance athletes; superimposed blunt or penetrating trauma; and presence of underlying comorbidities that could contribute to poorer healing, such as gout, collagen vascular disease, steroid intake, renal failure, etc. In the latter case with underlying chronic comorbidities, tendinosis can be much more pronounced, and tears may develop spontaneously due to poorer healing. To summarize, both theories of impingement and fatigue failure may play roles in rotator cuff pathology, and are not exclusive mechanisms. The “impingement” could be primary (abnormal subacromial anatomy) or secondary (due to abnormal GH motion), whereas “fatigue failure” likely plays an important role in the initiation or progression of RC tears, which can be further impacted by worsening impingement anatomy, as the tears progress. Patients with RC pathology usually present with weakness, night pain, and lateral shoulder and arm pain. In suspected RC injury or failure, the commonly used clinical tests include the above described impingement (Neer and Hawkins) tests; “Apley Scratch Test,” in which the person tries to touch the superior and inferior surfaces of the opposite scapula to assess the loss of range of motion; and the “Drop Arm Test,” in which the elevated arm is slowly lowered to the waist.
A common imaging finding in young weight lifters and overhead throwers is the so-called “rim-rent” tear. The term “rim-rent” tear (widely used by radiologists, although not so prevalent in the orthopedic community) refers to a partial articular-sided tear of the leading edge of the supraspinatus or infraspinatus tendons. The mechanism of such tears is explained below. With repeated or excessive overhead activities and underlying or developmental impingement anatomy, as the RC tendons slides under the hypertrophied AC joint, thickened coracoacromial ligament, low-set acromion, and/or subacromial spurs, there is repeated rubbing of the cuff with this altered subacromial impingement morphology leading to bursal surface scuffing and/or fraying. The latter is uncomfortable and/or painful and leads to further, uncoordinated (jerky) movements during shoulder abduction and external rotation. The subacromial impingement anatomy indents the myotendinous junction of the RC creating a pulley effect, which predisposes the RC tendon fibers to tear and avulse from the undersurface attachment at the greater tuberosity, leading to “attachment tear” a.k.a “rim-rent tear.” This mechanism also explains the well-known Burkhart’s concept of RC “cable and crescent” model. The crescent refers to peripheral attachment fibers of the RC on the greater tuberosity extending approximately 14 mm in transverse width. The cable is the thickened layer of the RC with a fibrous band that courses along the undersurface of the supraspinatus and infraspinatus tendons and blends with the CHL, at around 12 mm medial to the crescent and immediately lateral to the myotendinous junction. It was previously hypothesized that, in younger patients, the crescent plays the primary biomechanical role in RC function, but with age, and as the crescent weakens, the cable undergoes hypertrophy and assumes an important role in shielding the crescent from stress. Similar to a suspension bridge, the crescent tears first, and since few fibers (wires) avulse at a time, there is a nonuniform extension of the tear from anterior to posterior width of the rotator cuff. The crescent tears basically reflect “rim-rent” type of tears. Although partial-thickness tears may partially fill with fibrous tissue to some extent, more commonly, with advancing age, healing capacity lags and these tears progress. Therefore, partial tear, if left untreated, progresses to full-thickness tear, (best seen on coronal and axial images); and also increase in anteroposterior extent, progressing from incomplete to complete width, (best identified on the sagittal images). The normal cable should not be mistaken for partial articular-sided tear. On the other hand, a prominent cable appearance (abrupt contour change of supraspinatus articular surface) indicates the presence of a partial articular-sided tear.
RC tendinosis should be classified as mild, moderate, and severe to provide the clinician a sense of the degree of normalcy or quality of tendon fibers, in case repair is attempted. Again, there are no set rules; however, mild tendinosis is identified as intermediate signal (not fluid-bright) with maintained fiber continuity. This should not be overdiagnosed or mistaken for artifactual increased signal in the critical zone of the supraspinatus tendon due to magic angle phenomenon. Moderate tendinosis is identified as intermediate signal and mild thickening (>6 mm) of the tendon. Severe tendinosis refers to near fluid-bright and moderately thickened tendon, where differentiation from coexistent tears becomes difficult due to diffuse increased signal (Fig. 36). The term “focal tendinosis” should be avoided, since any focal area of signal hyperintensity, even if not fluid-bright, most likely represents a tear filled in with granulation tissue or fibrosis. Additional findings which might help in identifying focal partial tears include, missing tendon fibers (laminar retraction due to tear at the attachment), missing tight or loose tendon surfaces (surface tears), prominent cable, and presence of subcortical cysts in the greater or lesser tuberosity with focally increased, near-fluid signal in the tendon fibers adjacent to the cysts. The cysts may reflect cartilage rests (displaced cartilage from the physeal scar), or commonly, the RC tear can be traced to the individual cysts. The mechanism involves fibers getting avulsed from the bony attachment site, leading to tear of the enveloping synovium, which then results in filling of the cyst(s) by synovial fluid (or injected contrast). The various causes of RC tears include primary extrinsic impingement, secondary extrinsic impingement, internal impingement, regional bony derangements and tendinopathy related to systemic causes, apart from local direct or indirect trauma.
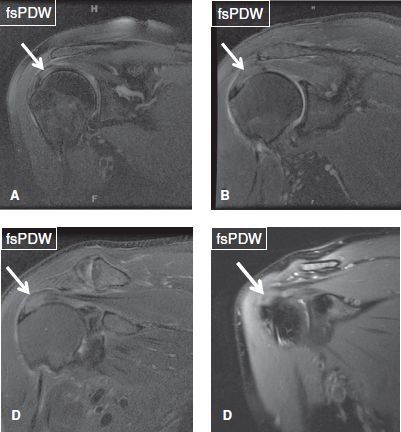
Fig. 36: Rotator cuff tendinosis. Arrows indicate mild (A), mild to moderate (B), moderate (C), and severe (D) rotator cuff tendinosis.
Calcific tendinitis is a self-limited painful disorder resulting from deposition of calcium hydroxyapatite crystals on the surface or within the substance of the RC tendons, most commonly of the supraspinatus tendon. Evident on radiographs as calcifications varying from a few millimeters to several centimeters in size, these crystals appear on MR images as rounded lesions of low signal on all pulse sequences, and are particularly conspicuous on gradient echo images. These are commonly located within 1 cm from the tuberosities. The natural history of calcific tendinitis usually follows the below described phases:
1. The silent phase, in which patients experience minimal or no symptoms, and radiographs demonstrate well-defined calcified deposits (Fig. 37).
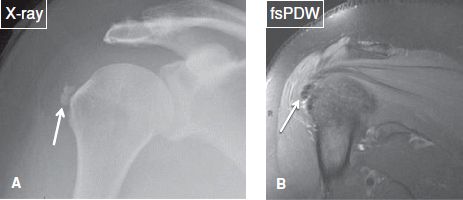
Fig. 37: Silent phase of calcific tendinitis. A: Anteroposterior radiograph demonstrates two rounded calcifications adjacent to the greater tuberosity (arrow). B: On the corresponding coronal MR image, the calcified bodies are depicted as hypointense lesions (arrow).
2. The active phase, in which patients experience impingement-like symptoms. The calcified deposits disperse into the adjacent bursa or peribursal soft tissues, and either disappear or become ill-defined on radiographs, whereas bursitis and/or inflammation of peribursal soft tissues are evident on MR images (Figs. 38–42).
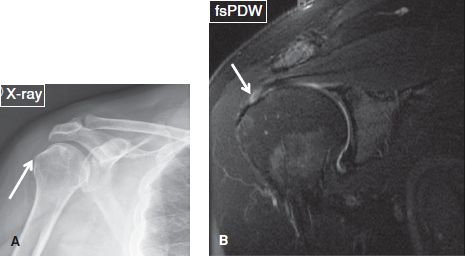
Fig. 38: Calcific tendinitis. A: Anteroposterior radiograph shows a calcification (arrow) adjacent to the greater tuberosity, corresponding to calcific tendinitis. B: The respective coronal MR image exhibits a high-grade bursal-sided partial tear (arrow) in the distal supraspinatus tendon.
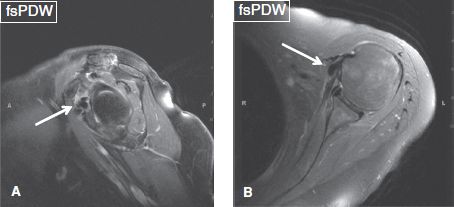
Fig. 39: Calcific tendinitis of the subscapularis muscle. Silent phase. Sagittal (A) and axial (B) images show hypointense lesions corresponding to calcific deposits (arrows) in the distal subscapularis tendon.
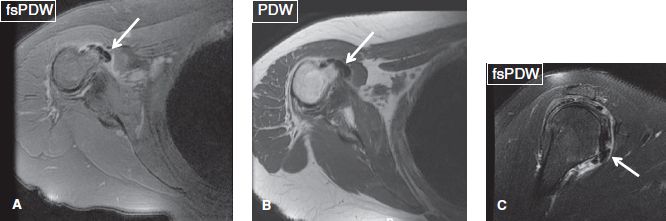
Fig. 40: Calcific tendinitis of the subscapularis muscle. Axial (A, B) and sagittal (C) images show hypointense lesions, corresponding to calcific deposits (arrows), in the distal subscapularis tendon. Notice associated subcoracoid bursitis.
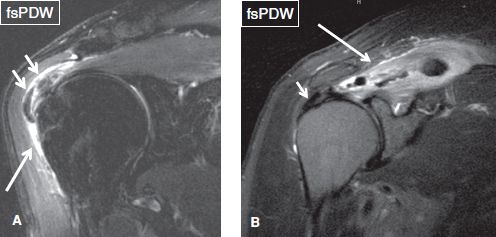
Fig. 41: Active phase of calcific tendinitis. Coronal images of different cases. A: Note moderate subacromial/subdeltoid bursitis (long arrow), as well as hypointense lesions (short arrows) adjacent to the greater tuberosity, corresponding to calcified deposits. B: Hypointense lesion (short arrow) adjacent to the greater tuberosity corresponds to calcified tendinous deposits. Edema of the supraspinatus tendon (long arrow) reflects calcific myositis.
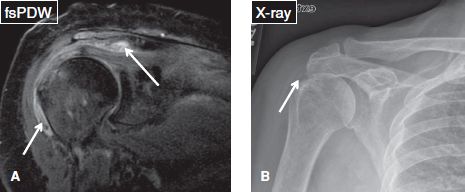
Fig. 42: Active phase of calcific tendinitis. A: Coronal image demonstrates moderate subacromial/subdeltoid bursitis with calcific debris (arrows). B: There is faint amorphous calcification on the anteroposterior radiograph (arrow).
3. The adhesive periarthritis phase, which is characterized clinically by debility, pain, and limited range of motion. Imaging modalities exhibit SASD bursitis changes, synovial thickening and calcified deposits within the RC and occasionally within the adjacent bone (Fig. 43).
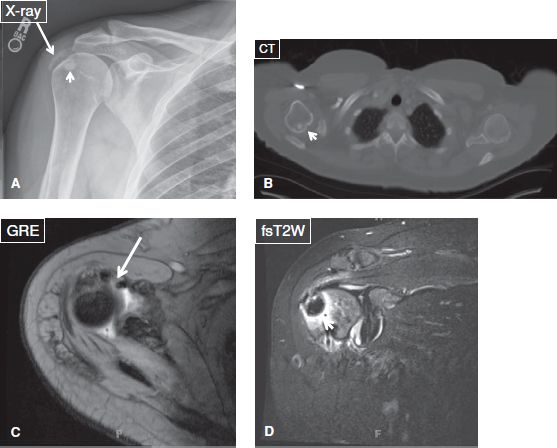
Fig. 43: Calcific tendinitis mimicking tumor. Anteroposterior radiograph (A), axial CT scan (B), and axial (C) and coronal (D) MR images demonstrate a relatively large calcified subarticular deposit (short arrows) within the humeral head, which can mimic a neoplasm. The adjacent calcified deposits (long arrows) within the rotator cuff confirm the case is calcific tendinitis.
RC tendon contusions from direct injury may mimic RC tears but can be differentiated from the latter by the absence of focal tendon contour changes, the normal position of the myotendinous junction, as well as by associated signs of trauma, such as bursal rupture, muscle strain, bony contusion, or fracture.
Partial RC tendon tears can be graded according to either the Ellman classification, which classifies tears based on their thickness and depth, into low grade (thickness <3 mm, involving <25% of RC thickness), intermediate grade (thickness 3 to 6 mm, involving 25% to 50% of RC thickness), and high grade (thickness >6 mm, involving >50% of RC thickness); or the Cofield classification, which classifies tears based on their anteroposterior width, into small (<1 cm), medium (1 to 3 cm), large (3 to 5 cm), and massive (>5 cm). Some orthopods use simple terminology, whether one tendon is torn or, two or more tendons are torn to decide their treatment approach. Snyder and Stetson developed a comprehensive classification that describes the tear location, size of the tear, and tendon quality for each RC tendon separately. In each RC tendon (supraspinatus, infraspinatus, subscapularis), the status of the articular (A) and bursal (B) sides is graded using a 5-scale scoring system (0 = normal, I = scuffing, II = fraying, III = partial-thickness tear, IV = near full-thickness tear). Full-thickness tears (C) are graded using a 4-scale scoring system (I = small full-thickness pinhole tear or <1 cm tear, II = 1 to 3 cm full thickness tear, III = 3 to 5 cm full-thickness tear, IV = >5 cm, complex/delaminating tear) (Figs. 44–57).
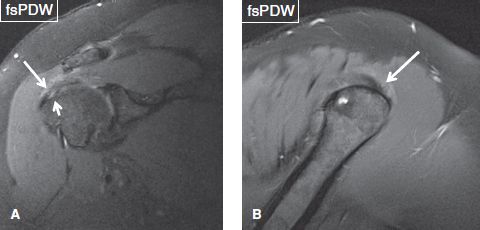
Fig. 44: Coronal (A) and sagittal (B) images demonstrate fraying of the bursal surface of the supraspinatus tendon (long arrows). Short arrow in (A) indicates a coexistent articular-sided, low-grade partial tear of the tendon attachment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







