Cartilage 
The articular surfaces of synovial joints are covered by hyaline cartilage, which protects the underlying subchondral bones by distributing loads and absorbing shock, maintains low-contact stresses, reduces friction and enables smooth motion of the articulating osseous structures. Damage to the hyaline cartilage usually results from everyday weight-bearing forces or sport activities and might be worsened with additional destabilizing pathology, such as tears (and, thus, subsequent loss of the supportive function) of menisci or labrum.
MR imaging is the method of choice to identify articular cartilage injuries and evaluate the post-operative reconstitution of cartilage repair tissue. Although various experimental techniques, such as T2 mapping, post-contrast T1 mapping, T1 rho imaging, glycosaminoglycan chemical exchange saturation transfer imaging (GAG CEST), and sodium MR imaging, are becoming feasible for the assessment of cartilage tissue architecture, conventional morphologic MR imaging remains the mainstay for the pre- and post-operative evaluation of the articular cartilage (Fig. 1). This chapter describes the structural anatomy of the articular cartilage, along with the respective normal and abnormal imaging appearances and delineates various grading systems and guidelines used in clinical practice for the imaging definitions of cartilage injury (chondrosis). In addition, surgical techniques for cartilage reconstitution are briefly discussed, and instructions are provided for filling in the structured template for the MR imaging description of repaired cartilage.
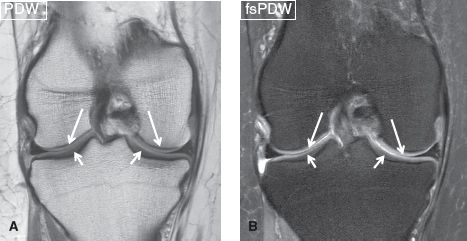
Fig. 1: Normal articular cartilage. Coronal images (A, B) of the knee demonstrate normal articular cartilage of the femoral condyles (long arrows) and tibial plateau (short arrows), featuring homogeneous thickness and a gradual increase in signal from the bone surface to the articular surface.
 ANATOMICAL ARCHITECTURE AND PATHOPHYSIOLOGY
ANATOMICAL ARCHITECTURE AND PATHOPHYSIOLOGY
The hyaline cartilage is a thin layer of soft tissue, wrapped around complex intra-articular anatomical structures. It is composed of 70% water, 20% collagen, and 5–10% proteoglycans and is very hypocellular since it is only composed of about 4% chondrocytes by wet weight. From superficial (near the joint fluid) to deep (near the subchondral bone), the cartilage is composed of (1) the lamina splendens, which is a thin hypointense protective layer; (2) a superficial layer of horizontal collagen fibers, which provide resistance against shear stress; (3) an intermediate/transitional layer of oblique fibers, which provide resistance against shear and compression stress; (4) a deep layer of radial fibers, which provide resistance against compression stress; and (5) a calcifying/tidemark zone, which tightly bonds the cartilage to the underlying bone.
The cartilage is subject to repetitive forces throughout life, and a normal adult loses 1% to 3% of articular cartilage thickness per year after 30 years of age, a process, which further worsens with the onset of osteoarthrosis or secondary insults from acute trauma, infection, or joint instability from meniscoligamentous injuries or inflammatory arthropathies. In general, arthritis-related cartilage defects show irregular and obtuse margins due to repetitive wear and tear whereas acute trauma-related defects are usually focal, isolated and demonstrate well-defined shouldered margins.
Cartilage damage progresses from chondromalacia (softening) to chondrosis (fissures, defects, flaps, delamination, and denudation on one side of the joint) and osteoarthrosis (cartilage defects or loss on both sides of the joint). Cartilage injuries are usually repaired by chondrocytes, which usually form fibrocartilage. The latter, however, is not very resilient in dealing with stresses as compared to the native hyaline cartilage. Full-thickness cartilage loss commonly results in stress changes in the underlying bone, causing pain and decreased range of motion in the affected joint.
On MR imaging, the normal five-layered configuration of hyaline cartilage is rarely visible, except in areas where it is thickest, for example, in patella. It is also only possible if high-resolution techniques combined with joint-specific coils and high-field strength (≥3 T) scanners are employed. On conventional (proton density-weighted [PDW] and fat-suppressed PDW [fsPDW]) imaging, the articular cartilage usually features a trilaminar configuration, composed of a low-signal deep layer (tidemark and radial zone), a thicker intermediate to hyperintense middle layer (oblique and horizontal fibers), and a thin low-signal surface layer (lamina splendens). In general, there is a gradual increase in signal from the bone surface to the articular surface. Additionally, the thickness of different layers of the cartilage vary for different surfaces of the articular bony margins (e.g., the radial layer is thicker in the weight-bearing central aspect of the tibia, and the transitional layer is thicker in the peripheral aspect) (Fig. 1).
 IMAGING OF ARTICULAR CARTILAGE PATHOLOGY
IMAGING OF ARTICULAR CARTILAGE PATHOLOGY
Cartilage injury is often a part of osteochondral lesions (OCLs). OCLs refer to one or two lesions on either joint surface, which have resulted from trauma, osteochondritis dissecans (OCD), or insufficiency fracture. More than two to three lesions are generally clubbed together as part of osteoarthrosis. MR imaging is the method of choice for the evaluation of both OCLs and intrasubstance lesions. A number of terms have been used for describing cartilage lesions on MR imaging, and multiple grading schemes have been proposed. Some of these include the Outerbridge Classification (1961), the Osteoarthritis Cartilage Histopathology Grading and Staging (OARSI, 2006), the International Cartilage Repair Society (ICRS) Classification (Table 1), and the Whole Organ MR Imaging Score (WORMS). Remembering and incorporating these ever-changing grading systems is a challenging task for both radiologists and referring physicians. In addition, on MR imaging, a number of lesions of varying severity may be identified, the interobserver variability tends to increase with complicated scoring systems, and a particular scoring system may not be efficient in classifying all such lesions. It is, therefore, better to describe the lesions in each compartment of a joint using accurate terminology rather than trying to fit all lesions into a particular scoring system. The terminology used in common clinical practice for describing cartilage lesions is illustrated in the following paragraphs.
TABLE 1: The International Cartilage Repair Society (ICRS) classification

Signal Heterogeneity
On MR imaging, signal heterogeneity may be caused by chondromalacia, fibrocartilage formation, cartilage degeneration, or mineralization. In most circumstances, chondromalacia (blistering or softening) is the earliest stage of cartilage injury; although it may be present in the cartilage either focally or diffusely in combination with higher grades of injury. The lesion usually involves the deeper layers of the cartilage and is likely related to fluid imbibition from a not-so-well seen surface fissure. The name derives from soft “malacic” feeling on arthroscopic probing. MR imaging may show focal area(s) of increased fluid-like T2 signal intensity in the deeper layers of the cartilage, with or without associated focal cartilage swelling. It can be differentiated from fissure and defect from the fact that the overlying lamina splendens hypointensity remains preserved. More commonly, the chondromalacic cartilage features diffusely increased signal with loss of the normally observed gradual deep to superficial signal alteration as described above (Figs. 2, 3).
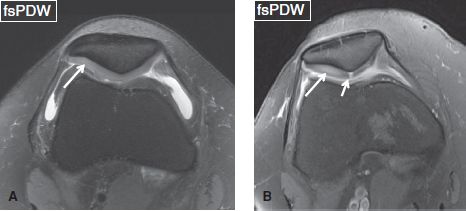
Fig. 2: Patellar chondromalacia. Axial images from different cases show diffuse hyperintensity (long arrows) of the lateral patellar facet cartilage (faint in A, prominent in B), which is indicative of chondromalacia. In (B), an additional full-thickness shouldered cartilage defect (short arrow) can be seen at the median patellar ridge.
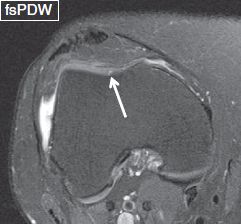
Fig. 3: Trochlear chondromalacia. Axial image reveals a tiny blister-like increased signal (arrow) at the central aspect of the trochlear cartilage.
Fibrocartilage formation, cartilage degeneration, and/or chondrocalcinosis are the result of subacute/chronic cartilage injury, as the cartilaginous tissue attempts to self-heal by fibrosis or mineralization. MR imaging shows focal or diffuse areas of low signal intensity within the articular cartilage (Figs. 4, 5). It is often difficult to differentiate fibrocartilage from chondrocalcinosis although the latter tends to be more focal and punctate and may additionally show blooming artifact on gradient echo images (Fig. 6). Correlation with plain films may assist in identifying the calcifications, although the lesions are often not visible, due to the low sensitivity and poor soft tissue contrast of conventional radiographs. Large areas of chondrocalcinosis may mimic incomplete discoid meniscus or vacuum in the joint (Fig. 7).
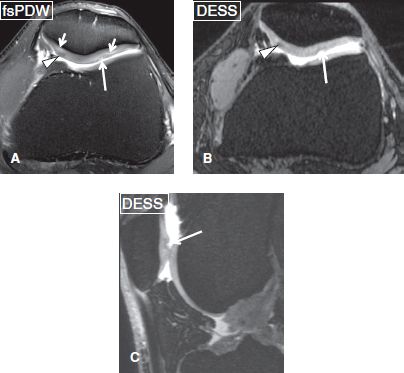
Fig. 4: Multiple patellar cartilage abnormalities. Axial image (A) exhibits linear areas of hyperintensity (short arrows) at the cartilage of both facets, which are indicative of chondromalacia, a hypointense focus (arrowhead) in the medial facet cartilage, which corresponds to fibrocartilage, as well as low-grade cartilage defects (long arrows) at the lateral facet. Axial (B) and coronal (C) reformations from a three-dimensional dual echo steady state sequence render the fibrocartilage focus (arrowheads) and cartilage defect (arrows) more prominent. Chondromalacia is less well seen on these 3D images.
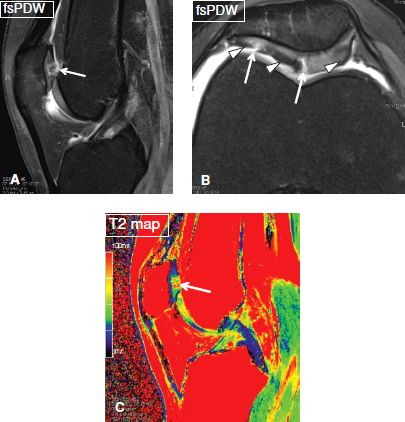
Fig. 5: Multifocal patellar chondrosis and signal alterations: Sagittal (A), axial (B), and corresponding sagittal color-coded T2 map (C) show multifocal high-grade patellar cartilage defects (arrows) with background areas of chondromalacia and fibrocartilage formation (arrowheads), not an uncommon finding in patellar malalignment cases.
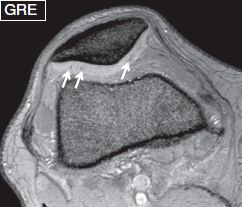
Fig. 6: Chondrocalcinosis. Axial gradient echo image exhibits punctuate foci (arrows) of low signal within the patellar cartilage, which correspond to calcifications.
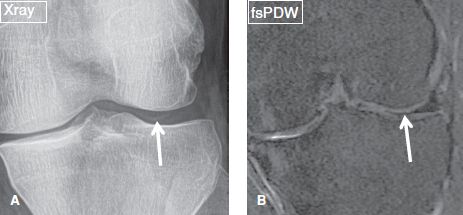
Fig. 7: Chondrocalcinosis. A: Anteroposterior plain film exhibits reveal linear opacity (arrow) in the lateral joint space, in keeping with chondrocalcinosis. B: On the corresponding coronal MR image, this chondrocalcinosis mimics incomplete discoid meniscus (arrow).
Partial-thickness Lesions
Partial-thickness defects qualify under chondrosis, and the term chondromalacia should not be used to describe them. These lesions can show a variety of morphologic appearances such as surface fibrillation, fissures, flaps, defects, or simply thinning. Apart from using these terms, one should describe whether the lesion is less than or more than 50% in thickness, namely low grade or high grade, respectively. If uncertain, one can use the term intermediate grade instead. Surface fibrillation represents an early stage of cartilage loss. On MR images, the cartilage shows minimal surface irregularity (fraying) and loss of the dark layer of the lamina splendens (Fig. 8). Cartilage fissure or flap results from further surface damage. Fissures are identified as tiny linear T2 bright gaps of less than 2 mm in transverse width, which assume a nearly perpendicular orientation to the articular surface (Fig. 9). A flap is formed by an obliquely oriented fissure, which causes elevation of the superficial (<50%) or deep (>50%) semi-separated layers of the articular cartilage (Figs. 10, 11). Cartilage defects are larger gaps with transverse width of greater than 2 mm. Based on the involved thickness, they can also be classified as low- and high grade, similar to fissures and flaps (Figs. 4, 5). In general, acute–subacute trauma related defects are well defined with shouldered margins, show acute angles and are fewer in number and are limited to the trauma sites. The osteoarthritis (OA)-related defects show obtuse margins, are ill-defined and occur at all sites, especially worse in the weight-bearing areas. Cartilage thinning appears as diffuse decrease of the cartilage thickness, with or without focal defects. In an individual joint, comparison with the cartilage thickness of the normal-appearing compartment assists in identifying the abnormality. Sometimes the cartilage may show low-intermediate grade thinning; however, presence of bony changes of sclerosis, cysts, and edema may indicate underlying full-thickness fissuring (Figs. 12–14). Patellofemoral pain syndrome patients will classically show patellofemoral cartilage abnormalities at the opposing surfaces with associated underlying friction-related Hoffa’s fat pad edema (Fig. 15). In addition, these subjects might show excessive or isolated cartilage loss of the far posterior medial or lateral femoral condyles in the tibiofemoral compartments (Fig. 16).
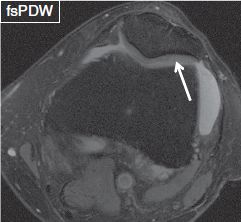
Fig. 8: Cartilage surface fibrillation. Axial image demonstrates minimal surface irregularity, thinning and loss of the dark layer of the lamina splendens (arrow) at the cartilage of the lateral patellar facet. Notice underlying chondromalacia of the lateral facet. This is already at the stage of chondrosis.
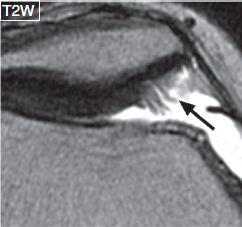
Fig. 9: Cartilage fissures. Magnified axial image demonstrates multiple high-grade cartilage fissures (arrow) at the medial patellar facet.
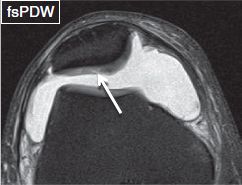
Fig. 10: Cartilage flap. Axial image shows an obliquely oriented fissure (arrow), which causes elevation of the superficial layers of the patellar cartilage.
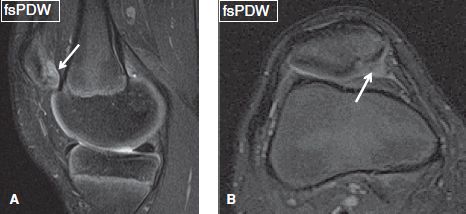
Fig. 11: Cartilage injury caused by transient patellar dislocation. Sagittal (A) and axial (B) images exhibit contusion and a high-grade flap (arrows) at the medial patellar facet cartilage with subchondral bony defect.
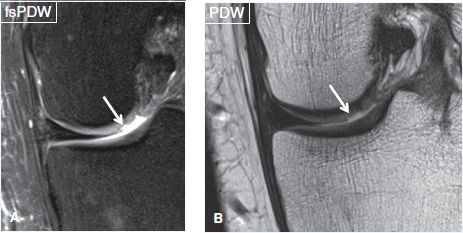
Fig. 12: Trauma-related shouldered cartilage defect. Coronal images (A, B) reveal a well-defined shouldered defect at the lateral aspect of the medial femoral condyle (arrows).
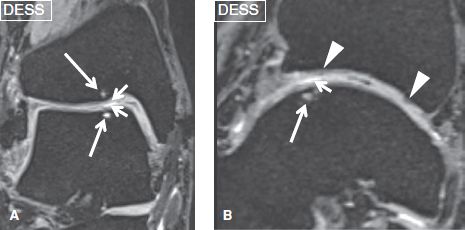
Fig. 13: Osteoarthritis-related cartilage loss. Coronal (A) and sagittal (B) reformatted images from a three-dimensional sequence show low- to intermediate-grade cartilage defects (short arrows) at the anterior aspect of the talar dome and the opposing surface of the tibia, as a result of chronic injury and resulting OA. Underlying subchondral cysts (long arrows) are presumably caused by thin, nonvisible fissures. Also note the foci of fibrocartilage (arrowheads) formation of the tibial articular cartilage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







