42 Key Points 1. Transcranial magnetic stimulation 2. Corticomuscular coherence 3. Galvanic stimulation 4. Auditory startle reactions 5. Treatment of spasticity Optimal individualized intervention following lesions in the central nervous system requires a detailed understanding of the structures and pathways that have been damaged in the individual patient. Furthermore, plastic changes occurring spontaneously or in response to intervention must be monitored to evaluate the efficiency of treatment. These plastic changes involve structures above the lesion, the spinal neuronal circuitries below the lesion, and the interaction between supraspinal and spinal circuitries. Most of these plastic changes are not apparent anatomically and are therefore out of reach for imaging techniques. The anatomical extent of a spinal lesion is also often not well correlated to the functional deficit.1 Physiological techniques by which transmission in specific spinal and supraspinal pathways may be evaluated are therefore of paramount importance for the evaluation of the underlying mechanisms of plastic changes and recovery of function following spinal cord lesions. This chapter reviews the present state of these techniques and sketches their use in evaluation of plastic changes in the nervous system in relation to (1) recovery of function following spinal cord lesion and (2) the development of spasticity. The use of transcranial magnetic stimulation (TMS) to evaluate transmission in surviving corticospinal tract fibers following spinal cord lesion is reviewed in other chapters. Here, it should only be added that the latency and amplitude of motor evoked potentials (MEPs) recorded at rest or during weak static voluntary activation of the target muscle, which is the normal standardized procedure, may not provide a fully functionally relevant measure of the subject’s ability to use the remaining corticospinal connectivity to control the full range of voluntary movements. It should therefore be noted that techniques are now available that allow the use of TMS during functional motor tasks, such as standing,2 walking,3,4 and even jumping and hopping.5 TMS may also be used during these motor tasks to condition Hoffmann reflexes (H-reflexes) (Fig. 42.1A) and in this way obtain specific information of task-related changes in activity of specific corticospinal pathways to the spinal motoneurons.4 This kind of information has the potential to provide a more specific and more functionally relevant indication of plastic changes in the recruitment of corticospinal pathways for control of specific tasks following spinal cord lesions and may turn out to be more optimal physiological outcome measures than the routine MEP measurements in sitting subjects. However, such measurements are not easy to do, especially in a routine clinical setting, and TMS also has the drawback that it involves an external stimulation of the corticospinal system and thus does not necessarily reflect the (unperturbed) normal neural activity in the pathway. Coherence and cross-correlation of electroencephalographic (EEG) and electromyographic (EMG) signals therefore provide attractive alternative techniques for evaluation of corticospinal transmission.6,7 With these techniques, events in different biological signals (such as EEG or EMG) that are time- or phase-locked to each other may be revealed. Activity in the motor cortex measured by EEG and in a voluntarily activated muscle measured by EMG thus show coupling in both the time and frequency domain reflecting the corticospinal activation of the muscle and the resulting sensory feedback.8 This corticomuscular coupling has also been shown to be greatly reduced or absent following lesion of both descending and ascending pathways in patients with stroke.9 The appearance of corticomuscular coupling also coincides closely with the development of the corticospinal tract and the acquisition of an adult-like control of fine finger movements during childhood.10 Different motor unit populations within the same muscle or in synergistic muscles show central short-term (lasting 10 to 20 msec) coupling in the time domain and characteristic peaks of coherence around 10 Hz and 15 to 35 Hz during voluntary movements.11 There is good evidence to believe that shared synaptic drive from corticospinal tract neurons is responsible for these features.6,11 Both the central short-term peaks of synchronization in the time domain and the 15 to 25 Hz coherence peaks have been shown to be reduced or absent in patients with stroke as well as patients with spinal cord injury (SCI).12,13 Cross-correlation and coherence techniques have the disadvantage that they require collaboration of the subject and cannot be used if the subject has no remaining voluntary effort. However, the techniques are simple and may easily be performed in a routine clinical setting because they require only a couple of minutes of paired surface EEG or EMG recordings. Furthermore, new analysis techniques allow measurements during functional motor tasks like walking14 and reaching15 to also be obtained. It has thus been demonstrated that healthy subjects show a characteristic modulation of coupling of populations of motor units during gait, which likely reflects the contribution of descending drive to the activation of the muscles.14 This coupling is greatly reduced or absent during gait in SCI patients and on the injured side in stroke patients.16,17 It shows a very good correlation to the functional deficit in the patients, and it appears to be a good marker of functional recovery in relation to gait training in SCI patients.18 These features and the ease of its use make this an optimal method for physiological outcome evaluation in relation to assessment of interventions in SCI patients. Fig. 42.1 (A) Transcranial magnetic stimulation, (B) galvanic stimulation, and (C) audiospinal stimulation can be used to modulate soleus H-reflex (D) to investigate corticospinal, vestibulospinal, and reticulospinal tracts, respectively. The role of descending motor tracts other than the corticospinal tract for functional recovery following SCI has been somewhat neglected, probably mainly due to the relative inaccessibility of these tracts for electrophysiological testing. However, in recent years techniques have been developed that appear to provide some information of the conduction in the vestibulospinal and reticulospinal tracts. This information is likely to be of great importance for an understanding of the recovery of posture, balance, and gait following SCI. Galvanic stimulation (Fig. 42.1B) involves application of a small constant electric current (around 1 mA) between electrodes placed behind the ears on the mastoid processes, which elicits enhanced body sway in the direction of the ear behind which the anode is placed.19 The muscular responses evoked by the stimulation in leg muscles are in all likelihood mediated by transmission in the vestibulospinal tract20 and appear to provide valuable information about altered transmission in the tract following stroke21 and SCI.22 Auditory stimulation or startle reactions (Fig. 42.1C) are in all likelihood generated by reticulospinal tracts from the brain stem.23 They have been shown to be hyperactive in muscles rostral to a spinal cord lesion, possibly as a plastic adaptation to the impaired control of muscles below the lesion.24 Startle reactions are also exaggerated in cortical stroke patients.25 There is evidence in rats that assessment of auditory startle reactions may be useful in evaluating the extent of lesion of reticulospinal tracts,23 but this is of limited use in human subjects, where startle responses are seldom seen in most distal arm and leg muscles. However, startle stimuli have been shown to trigger patterned movements in various muscles, including leg muscles during standing.26 In all likelihood these responses are also mediated by reticulospinal projections and may therefore possibly be used to assess reticulospinal transmission across spinal injuries. It should also be noted that startle reactions may be observed in leg muscles also in relation to functional motor tasks.27 Modification of the H-reflex (Fig. 42.1D) by TMS, galvanic stimulation, and audiospinal stimulation thus provides a way of studying transmission in corticospinal, vestibulospinal, and reticulospinal tracts, respectively. Changes in transmission in spinal neuronal pathways have been investigated following lesion of descending motor pathways in numerous studies since the beginning of the 1970s.28,29 The focus of most of this research has been on the possible contribution of changes in inhibitory spinal mechanisms to the development of spasticity with the perspective of finding target points for therapeutic interventions.30 Alteration in several spinal control mechanisms has been demonstrated, including presynaptic inhibition,31,32 postactivation depression,32 disynaptic reciprocal inhibition,33,34 and persistent inward currents in the motoneurons.35 From this research it appears clear that no single mechanism explains the development of spasticity, but that alteration in several interdependent mechanisms involving different neuronal pathways is likely to be involved. The relative changes in these different mechanisms have been shown to depend on several factors, which vary between subjects and determine the severity of spasticity relative to the subject´s functional ability. Spasticity should thus be seen as a plastic adaptation to the altered activity in the spinal neuronal circuitry secondary to the lesion of the descending pathways and secondary to the altered activity pattern in surviving descending motor tracts and sensory afferents. Short- and long-lasting plastic changes in the mechanisms that are involved in spasticity have thus been demonstrated in relation to immobilization36 and the extent and type of exercise.37 With this degree of modifiability of the mechanisms involved in spasticity, it seems within reach to find interventions that may help the surviving descending motor tracts to make more optimal use of the altered spinal neuronal circuitries and thus promote functional recovery. There is indeed an increasing understanding that weak to moderate spasticity may not be a “bad” thing and that spasticity may often be used by the patient to perform voluntary movements.38 Rather than to simply remove spasticity, the goal of “antispastic” treatment should therefore in many cases be to find ways to help the patient—and the surviving descending tracts—to make new use of the altered settings in the spinal circuitries. Research into how the altered spinal circuitries may contribute to spontaneous recovery of function and how different interventions may make use of the altered spinal circuitries and induce changes in them and their descending control has already been conducted in relation to gait training,39,40 and more research will undoubtedly appear when the full potential of this approach is generally realized. Pearls
The Role of Neurophysiology in the Study of Recovery and Spasticity
 Evaluation of Transmission in the Corticospinal Tract
Evaluation of Transmission in the Corticospinal Tract
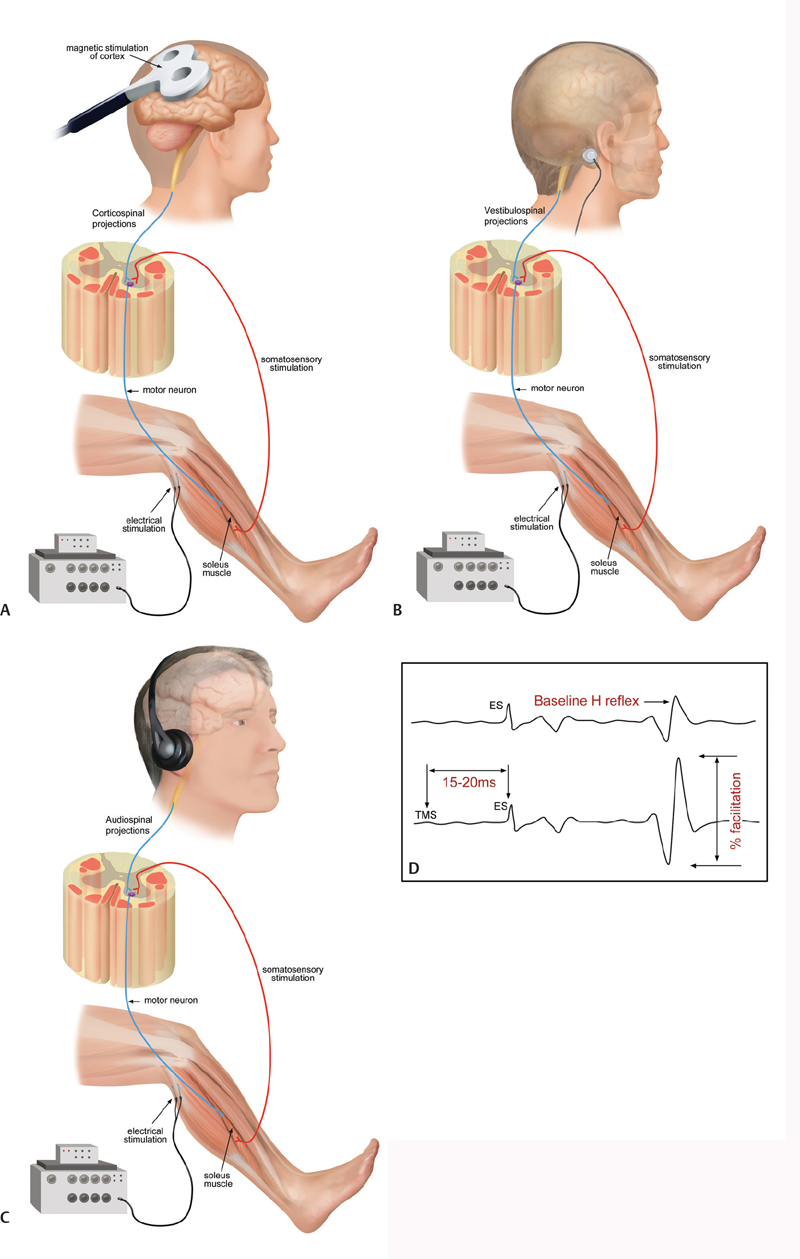
 Evaluation of Transmission in Descending Motor Tracts from the Brain Stem
Evaluation of Transmission in Descending Motor Tracts from the Brain Stem
 Evaluation of Transmission in Spinal Neuronal Pathways in Relation to Spasticity
Evaluation of Transmission in Spinal Neuronal Pathways in Relation to Spasticity
 Electrophysiological techniques provide information about functional transmission, adaptation, and reorganization of specific supraspinal and spinal neural pathways that is inaccessible with other techniques.
Electrophysiological techniques provide information about functional transmission, adaptation, and reorganization of specific supraspinal and spinal neural pathways that is inaccessible with other techniques.
 Coherence and cross-correlation analysis of biological signals provides information about the functional organization and reorganization of unperturbed neural networks during natural movements.
Coherence and cross-correlation analysis of biological signals provides information about the functional organization and reorganization of unperturbed neural networks during natural movements.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine
