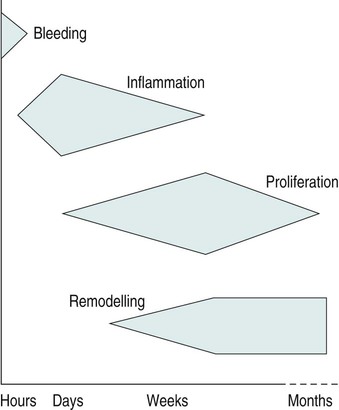
• to identify the aims of physiotherapy and how they change throughout the continuum of tissue healing and repair; • to provide the reader with specific physiotherapy examples of managing inflammation and repair; • to develop the reader’s ability to clinically reason the use of core physiotherapy modalities in the management of tissue healing and repair; These factors may significantly alter the approach a clinician takes and maybe even exclude certain treatment modalities that would normally be considered. It is this individualised, holistic and evidence-based approach that makes the physiotherapist an invaluable contributor to the patient’s optimal recovery from disease or injury. The clinical reasoning process is illustrated in Figure 12.1. This process starts with a short period of tissue bleeding owing to the disruption of small blood vessels and capillaries. Immediately following this period of bleeding a complex cascade of biochemical events proceed, triggering an inflammatory reaction. The inflammatory process initiates the proliferation of new tissue cells, which eventually remodel with the aim of restoring normal tissue function. This sequence of events is illustrated in Figure 12.2. When injury or trauma occurs producing forces necessary to damage soft tissues it is important to consider what happens to these tissues at a cellular level. The secondary injury model described by Merrick (2002) considers there to be two stages following tissue injury, namely the primary and secondary injury response: • the primary injury is considered to be the damage to cells caused by the direct injury mechanism, be that a crush, contusion or strain force. The cells damaged by this mechanical force may have their cell membranes disrupted causing a loss in homeostasis and subsequent cell death. Many types of tissue may be involved, including ligament, tendon, muscle, nerve and connective tissues; • secondary injury is thought to be caused by physiological responses to the initial primary injury. This occurs to tissues at the periphery of the injury to those cells not damaged by the primary injury. It is hypothesised that this damage may occur by two means. These are hypoxic (or maybe better described as ischaemic) and enzymatic mechanisms. This is a relatively short phase that will depend upon the initial injury type, the tissues damaged and the severity of injury. If there has been an injury to soft tissues some degree of bleeding will occur. When capillaries and small blood vessels sustain primary injury, blood will escape into surrounding tissues and, depending on the location, may gradually track distally as a result of gravity. The type of tissue involved and the type of injury will determine the degree of bleeding in terms of amount and duration. If very vascular tissue is damaged (e.g. muscle) a larger amount of bleeding will occur in comparison with less vascular tissues, for example ligament and tendon. The bleeding phase may only last a period of a few minutes or hours, but in large muscle contusion injuries, for example, bleeding may continue to a small degree for up to 24 hours (Watson 2004). Following an injury or pathological process there is an immediate inflammatory response. Scott et al. (2004) describe inflammation as a complex process that can be viewed at four levels: clinical, physiological, cellular and molecular, and suggest that it is the application of clinical reasoning skills that allows us to define which level is relevant in any given situation. Discussion of the complex biochemical sequences involved in the inflammatory process are beyond the scope of this chapter – the reader is referred to the wealth of books which discuss these biochemical and pathological aspects in more detail. The inflammatory response has been reported to last for several days, or even weeks, but usually peaks around 2–3 days post-injury. During this inflammatory phase there may well be several clinical features evident. These include redness, swelling, pain and loss of function. During inflammation there is primarily a vascular and cellular response. The vascular response is a result of chemical inflammatory mediators and also a neural effect on the arterioles. There is an initial vasoconstriction that lasts only a period of seconds, followed by a more prolonged vasodilation response. There is also an increase in the permeability of the capillary walls allowing migration of large plasma proteins into the interstitial space. This alters the osmotic pressure in the tissue and exudate will gather in the interstitial space causing swelling. As cells migrate across the vessel wall into the interstitial fluid this will become cellular exudate. This exudate will contain mainly neutrophils initially and then lymphocytes and monocytes as the inflammatory process progresses (Mutsaers et al. 1997). • to limit and reduce inflammatory exudates; • to reduce metabolic demands of tissue; • to protect newly damaged tissue from further injury; • to protect the newly-forming tissue from disruption; • to promote new tissue growth and fibre realignment; • to maintain general levels of cardiovascular and musculoskeletal fitness/activity. These interventions together are applied in principle to address the seven aims of early phase tissue injury and healing management. These interventions are discussed below. The reader is also referred to the guidelines written in conjunction with the Association of Chartered Physiotherapists in Sports Medicine (ACPSM), on the immediate management of soft tissue injury using the PRICE principles (Bleakley et al. 2010). However, it must be remembered that excessive unloading of the structures and prolonged rest can do harm to the patient so must be balanced carefully with appropriate activity (Bleakley et al. 2010). Therefore, the physiotherapist needs to carefully balance these protection and rest aims with those of promoting movement in order to maintain normal function of adjacent joints and structures. As this very early phase ends (around 48 hours), very gentle movement is needed to help improve circulation and removal of waste products, and to provide the necessary stresses for the correct alignment and orientation of newly forming tissue fibres. Both local and systemic exercise can assist in this process through changes in haemodynamics and lymphatic function (Bleakley et al. 2010). The primary reason for applying ice in the immediate early injury management is to cool the affected tissues, hence reducing the metabolic demands of the neighbouring cells. This should enable more cells to survive the ischaemic phase, thus minimising secondary tissue damage. It is suggested that to maximise the therapeutic effects of cryotherapy, an optimal tissue temperature reduction of 10–15° is required (MacAuley 2001). If the application of cryotherapy can reduce the number of cells damaged overall, the healing and repair process will be quicker, hence speeding up return to function. Traditionally, the application of cryotherapy may have been thought to induce vasoconstriction of the small blood vessels, thus reducing blood supply to the area and hence causing increased ischaemia to the tissues and further secondary damage as initially described by Knight (1989). Merrick (2002) suggests that the secondary injury model described above now better describes the effects of cryotherapy. It must be noted, however, that there is very little conclusive evidence that investigates how cryotherapy affects the metabolic processes and whether it can influence inflammation (Bleakley et al. 2010). The duration, application and frequency of cooling to achieve maximum therapeutic benefits has not yet been determined scientifically, yet these factors will greatly affect the degree of tissue cooling. Not all modes of cooling are equally effective; however, crushed ice provides effective cooling and is probably the safest (Bleakley et al. 2010). Subcutaneous fat covering will insulate deeper tissues and thus limit cooling significantly. Therefore, some areas may require slightly longer cooling times. Other important points are that the cold application should cover the whole area of injured tissue and a damp towel should be placed between the cooling agent and the skin to avoid skin and tissue damage. Using a thick, dry protective layer is likely to significantly reduce the cooling effects. It is also worth noting that research suggests that deep tissue temperature stays the same or continues to cool for up to 10 minutes following removal of an ice pack (Bleakley et al. 2010). The theory behind the application of compression is that the hydrostatic pressure of the interstitial fluid is raised, thus pushing fluid back into the lymph vessels and capillaries, and reducing the amount of fluid that can seep out into surrounding tissues (Rucinski et al. 1991). External compression through the application of an elastic wrap can stop bleeding, inhibit seepage into underlying tissue spaces and help disperse excess fluid (Thorsson et al. 1997). Unfortunately, there are very few studies which look specifically at the effects of applying compression alone in acute soft tissue injury and, again, much of contemporary practice is based upon experiential evidence and consensus opinion (Bleakley et al. 2010).
The physiotherapy management of inflammation, healing and repair
Introduction
The continuum of tissue healing and repair
Soft tissue injury
Phases 1 and 2 of tissue healing and repair
Phase 1: bleeding (0–10 hours)
Phase 2: inflammation (0–4 days)
Physiotherapy interventions in phases 1 and 2 (0–72 hours post-injury)
General physiotherapy aims of early phase management (phases 1 and 2)
The PRICE principles
Rest
Cryotherapy (ice therapy)
Compression