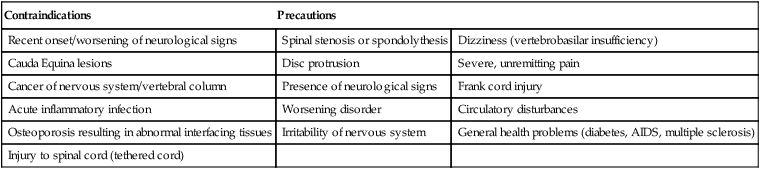
In 1864, Lasegue first described the straight leg raise test (SLR) as an assessment for lower back problems with nerve involvement (Butler 1991). This concept was further developed by Goddard and Reid in 1965 who described the SLR as movement of the sciatic nerve. Also, in the 1960s Alf Breig investigated the biomechanics of the nervous system in more detail and showed that nerves move independently from other tissues. This formed the basis of the concept of ‘neurodynamics’. Earlier terms for this concept were ‘neural tension’ (Breig 1978) or ‘adverse mechanical tension of the nervous system’ (Butler 1989). However, the actual term ‘neurodynamics’ was first introduced by Gifford in 1989. The concept of neurodynamics has been further developed by Grieve, Butler, Maitland and Shacklock over the last 30 years. Nowadays, although neurodynamics remains a relatively new concept within musculoskeletal clinical assessments, it is becoming more widely used and accepted as an important aspect for injury assessment and treatment. In general terms, the nervous system can be divided into the central nervous system (CNS; brain and spinal cord), peripheral nervous system (all nerves after leaving the nerve roots within the spinal vertebrae) and autonomic nervous system (sympathetic and parasympathetic division) (Michael-Titus et al. 2007). The central and peripheral nervous system are closely connected through meninges (Butler 1991). The mechanical and physiological properties of peripheral nerves are crucial in their ability to function well. The peripheral nerves are not only involved in conducting impulses and chemical traffic, but are also considered as dynamic tissue in the same way as muscles and joints. Along with the CNS, the peripheral nerves are designed to function while stretching and sliding (longitudinal and transverse motion) as a result of a wide variety and range of body movements (Shacklock 1995). When nerves are sliding they always slide towards the joint where movement takes place (Coppieters and Butler 2008). During movements of the body, nerves can undergo extremes of elongation, for example the spinal canal is 5–9 cm longer in flexion than extension, thus leading to an increased tensile load on the spinal cord (Breig 1978; Louis 1981 cited in Butler and Gifford 1989). Another example is the median nerve which has to adapt to a nerve bed nearly 20% longer if the shoulder is 90 degrees abducted, and the wrist and elbow are moved from flexion to extension (Millesi et al. 1986). The nervous tissues are able to tolerate large tensile and compressive forces during movements associated with daily living and sporting activities. Nerves are strong structures owing to the surrounding connective tissue sheath helping them withstand large compressive and tensile forces (Shacklock 1995). If nerves are loaded by movement they react by moving and absorbing some of the pressure. During normal movement, as the nerve elongates the perineurium tightens, intraneural pressure increases and intrafasicular capillaries stop flowing. This occurs at 8% elongation, with intraneural microcirculation ceasing completely at 15% elongation (Ogata and Naito 1986). As the nervous system is dynamic, forces that cause a temporary disruption are easily tolerated. However, if the load becomes too excessive or persists, the maintenance of the increased local pressure to the nerve can cause local tissue ischaemia and injury. Injuries involving nervous structures can either be caused by direct or indirect mechanics. A direct injury can occur from over-stretching or irritation from a repetitious activity, whereas an indirect injury can result from bleeding owing to a muscle tear through which the nerve passes. Either of these types of injury can cause extraneural dysfunction (affecting movements outside of the nerve; relative to the nerve) or intraneural dysfunction (affecting movements within the nerve (Butler (1989)). These two forms of dysfunction are not exclusive of one another and sometimes extraneural dysfunction can actually lead to intraneural dysfunction. Both, however, will be discussed separately in further detail. The term ‘extraneural’ refers to anything outside of the nerve. This refers to the ‘mechanical interface’, which is any structure surrounding the nerves and lying between them and other surrounding structures (Butler and Gifford 1989). The nervous system is surrounded by, and passes through or around, various structures, including muscle, bone, tendon, blood vessels and intervertebral discs. These help contain the nervous system and allow for its movement, so as the nerve shortens, elongates or twists during daily movement so does the mechanical interface (Shacklock 1995). The smooth and normal movement of the nervous system and mechanical interface as it interacts with the musculoskeletal system is important for injury prevention. The consequence of extraneural pathology is to subject the nerve to increased tensile, frictional or compressive load. Examples of vertebral extraneural pathology are the narrowing of the intervertebral foramina (e.g. through osteophytes) or disc protrusions irritating a nerve root. In the peripheral nervous system, examples of extraneural pathology include the sciatic nerve lying in a bed of blood from a hamstring tear, tight fascial bands across nerves and a nerve as it passes through an oedematous tunnel (tarsal tunnel, carpal tunnel). Intraneural dysfunction refers to within the nerve itself. Entrapment of a nerve by surrounding tissues or with an intraneural fibrosis (scarring within the nerve structures) can cause pathological changes within the nerve (Butler and Gifford 1989). This may lead to a loss of range of movement or alteration in elasticity of the nerve and, as a result, enhance the normal mechano-sensitivity of the nerve during movement and thus provoke pain and reactive muscle spasm to prevent elongation. The term mechano-sentitivity refers to how easily a nerve is activated following application of mechanical force (Shacklock 2005). There are a variety of causes for mechano-sensitivity; however, perhaps the most common factor is owing to pressure. This can either be applied directly or indirectly, as will be discussed. Direct mechanical pressure can be applied to the nerve by any tissue infringing the space occupied by the nerve (e.g. ‘bulging disc’ or poorly fitted athletic knee brace). For example, compression of a disc hernia reduces blood flow in the nerve by 70% (Kobayashi et al. 2003). When loading persists, as it could from sustained un-physiological postures or maintained local pressures, damage may ensue as a result of local tissue ischaemia. Double (DCS) or multiple crush syndrome (MCS) refers to a condition where proximal compression or elongation of a nerve decreases the nerve’s ability to withstand compression or elongation at a distal site (Upton and McComas 1973). This type of condition is caused by changes to the nerve’s axoplasmic flow. Axoplasmic flow is the term used to describe the transport mechanism of cell components in the nerve to their functional site where they help to maintain tissue health. Therefore, any pressure which is directly applied to the nerve or in the surrounding tissue following injury may cause slowing down of the axoplasmic flow and cause the nerve to ‘become sick’. If pressure is applied to two (double crush) or multiple areas (multiple crush), the nerve will be considerably more prone to injury. The original DCS described neural injuries at the neck which then predisposed to carpal tunnel syndrome (Upton and McComas 1973). Alternatively, reversed DCS occurs where a distal neural injury (e.g. at the wrist) predisposes to a proximal injury, perhaps at the shoulder. MCS occurs where following initial neural injury a patient experiences multiple and related areas of symptoms. Clinical presentation of DCS or MCS may often be widespread and unfamiliar in nature. However, patients may complain of a string of injuries or multiple areas of localised pain which have common pathophysiologies that represent a minor form of DCS or MCS. As well as indications, there are also contraindications and precautions to neurodynamic testing. Contraindications represent any kind of factor or condition that increases the risk or danger to cause serious harm to the client by carrying out neurodynamic testing. Therefore, if any contraindications are present the neurodynamic tests should not be carried out. Precautions are factors that you should be aware of and take additional care of when carrying out and interpreting neurodynamic tests, although this does not mean that you cannot carry out the tests. Common contraindications and precautions to neurodynamic testing are presented in Table 25.1 (Butler 1989). Table 25.1 Contraindications and precautions to neurodynamic testing Neurodynamic tests involve assessing the mobility of the nervous system as it interacts with the musculoskeletal system. However, this can create a problem when trying to distinguish whether reproductions of the client’s pain/symptoms during a test is as a result of the movement by nervous or musculoskeletal structures. Structural differentiation emphasises movement of the neural tissues as opposed to the musculoskeletal tissues, therefore helping to identify the damaged structure – whether neural or musculoskeletal (Butler 2000). For example, when performing the slump test by extending the knee it would cause movement, and potential tension, of both nervous (sciatic nerve) and musculoskeletal (hamstrings) tissues. To help distinguish which structure was the cause of the client’s pain/symptoms, we could get the client to release the cervical flexion. This structurally differentiating manoeuvre is unlikely to alter the strain on the lower limb musculoskeletal structures (hamstrings); however, it will change the tension on the nervous system. Therefore, if this manoeuvre resulted in reducing the client’s pain/symptoms, we could propose that their injury was related to neurological, rather than musculoskeletal, damage. While structural differentiation is a key element for helping identify neurogenic problems, for patients with a high level of function less severe problems may remain hidden. Therefore, in order to make the test more specific to identify the problem we can add sensitising manoeuvres. This is where the normal neurodynamic technique is modified slightly to increase the test sensitivity so that it becomes more specific to the patient’s problem (Keneally et al. 1988 ). The easiest and most common way of sensitising tests is to put extra strain, or stretch, on the nerve by elongating it. A simple example of this would be to add cervical flexion when performing a SLR. Another sensitising manoeuvre would be to contract a muscle close to the nerve pathway around the affected area during the neurodynamic test, such as contracting piriformis muscle during SLR. • the quality of the movement (e.g. is the movement smooth and not jerky); • the range of movement (similarity bi-laterally); • resistance through range and at end-of-range (here, the clinician may feel tightness that stops or impairs the movement); • pain and symptoms through range (this may or may not be specific to reproducing their pain/symptoms). • Optimal – this is where the neural system behaves exactly the way it should, allows full range of movement and does not produce any symptoms or discomfort to the patient, even when high loads and stress are applied. • Suboptimal – the neural system here behaves less well, allows slightly reduced range of movement and may result in producing some symptoms in the patient, although not necessarily until a high enough load is applied to provoke the system. • Normal – here, the patient is likely to have some symptoms during the testing; however, the function of the neural system (i.e. range of movement) is considered to be within ‘normal’ values (although these are not clearly established) or similar on both sides. • Abnormal – this is where the function of the neural system is obviously outside the normal range and the patient may have significant symptoms. This can be caused by pathology or abnormalities in the mechanics and physiology of the nervous tissues. Therefore, as abnormalities do not always produce symptoms, the ‘abnormal’ response can be further divided into:
Neurodynamics
Introduction
Mechanics of the peripheral nervous system
Pathophysiology of the nervous system
Extraneural dysfunction
Intraneural dysfunction
Mechano-sensitivity
Double and multiple crush syndrome
Assessment principles
Indications
Contraindications
Precautions
Recent onset/worsening of neurological signs
Spinal stenosis or spondolythesis
Dizziness (vertebrobasilar insufficiency)
Cauda Equina lesions
Disc protrusion
Severe, unremitting pain
Cancer of nervous system/vertebral column
Presence of neurological signs
Frank cord injury
Acute inflammatory infection
Worsening disorder
Circulatory disturbances
Osteoporosis resulting in abnormal interfacing tissues
Irritability of nervous system
General health problems (diabetes, AIDS, multiple sclerosis)
Injury to spinal cord (tethered cord)
Structural differentiation
Sensitising manoeuvres
Interpretation of findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neurodynamics




