22 Key Points 1. A severe spinal cord injury affects every major organ system. 2. Secondary complications lead to increased health care utilization and costs. 3. Specialized medical care and ongoing support are required to prevent secondary complications and maintain long-term health. 4. The management of the following medical complications is reviewed—heterotopic ossification, neurogenic bladder dysfunction, neurogenic bowel dysfunction, osteoporosis, respiratory management, spasticity, and upper extremity preservation. A spinal cord injury (SCI) is a devastating event. In addition to the accompanying loss of function and independence, the affected individual is predisposed to a constellation of secondary complications. A severe SCI affects every major organ system (e.g., pressure ulcers, neurogenic bowel and bladder dysfunction, sublesional osteoporosis, neuropathic pain, cardiovascular dysfunction), and individuals remain at risk for secondary complications for the remainder of their lives. The complications are distinct from those associated with normal aging. One study from the US Model SCI System found that 96% of clients had a medical complication when seen for routine annual follow-up.1 A Canadian study reported that 56% of individuals had experienced a urinary tract infection during the previous year, with another 28% reporting a pressure ulcer.2 Secondary complications ultimately lead to increased health care utilization and costs. Dryden and colleagues reported that, compared with controls, individuals with SCI were rehospitalized 2.6 times more often and were 2.7 times more likely to have physician contact during a 6-year follow-up period postinjury.3 Fifty-seven percent were hospitalized at least once during this period, with 32% admitted between three and nine times. Four percent were hospitalized on 10 or more occasions. Recent studies continue to confirm increased health care utilization following SCI. During the first year postdischarge, the mean number of physician visits was ∼ 30, and 27.5% of individuals required at least one rehospitalization.4,5 The responsibility of the health care system therefore does not end when the individual with SCI leaves the acute care or rehabilitation center to reenter the community. Health care providers must remain proactive and vigilant; and specialized and ongoing medical care is required to prevent secondary complications, maintain long-term health, and maximize overall health and quality of life. This chapter reviews the management of common complications following SCI. Autonomic dysreflexia (AD) is a condition characterized by episodes of malignant hypertension, which, when severe, can lead to potentially life-threatening complications, such as stroke. AD occurs in individuals with SCI at or above T6. It increases with ascending level and injury severity6,7 and is three times more common following complete compared with incomplete injuries.6 The pathophysiology and treatment of this condition are discussed in detail in Chapter 16. The prevalence of risk factors for coronary artery disease (CAD) increases following SCI. Specific risk factors include elements of the metabolic syndrome (hyperlipidemia, abdominal obesity, glucose intolerance, insulin resistance), elevated homocysteine, elevated C-reactive protein (CRP), and low physical activity/fitness levels. The prevalence of CAD risk factors translates into a higher incidence of CAD following SCI. Cardiovascular morbidity and mortality occur earlier and more often than in the able-bodied population. As a result, CAD is the leading cause of death for individuals living with chronic SCI.8 CAD can also be asymptomatic (“silent”). Visceral afferent fibers from the heart enter the spinal cord at T1–4, and individuals with neurological levels at and above these segments might not experience cardiac pain. The exact prevalence of asymptomatic CAD among people with SCI is unclear, with reported rates ranging from 25 to 65%.9 Because CAD can be relatively asymptomatic following SCI, the diagnosis requires vigilance and a high index of suspicion on the part of the clinician. A comprehensive review of cardiovascular health following SCI is provided in Chapter 16. Heterotopic ossification (HO) is a condition characterized by the formation of mature ectopic bone in the periarticular soft tissues of joints. HO occurs after SCI, traumatic brain injury, burns, and acetabular fractures. Although our understanding of the underlying pathophysiology is limited, the differentiation of primitive mesenchymal stem cells into osteogenic cells plays a prominent role. In the setting of SCI, HO always occurs at joints below the neurological level of the injury, with the most common site being the hips, followed in descending order of frequency by the knees, shoulders, elbows, and hands. Although severe cases can lead to a loss of range of motion and even joint ankylosis, most cases are asymptomatic and often diagnosed incidentally. Loss of hip range of motion can interfere with seated posture and independence during transfers. Signs of earlier HO are nonspecific and inflammatory—erythema, swelling, low-grade fever. Calcified HO is visible with plain x-ray but triple-phase bone scan remains the gold standard for the diagnosis of earlier HO prior to maturation and calcification. HO becomes evident on plain radiography ∼ 2 to 6 weeks after diagnosis using triple-phase bone scan.10,11 Early diagnosis is critical because treatment is more effective when initiated prior to the formation of visible calcification. Serum alkaline phosphatase rises prior to radiographic evidence of HO and may be used for monitoring the activity of HO. Urinary excretion of prostaglandin E2 has also been reported to be elevated in individuals with acute SCI who later develop HO.12 The mainstays of treatment are nonsteroidal antiinflammatories (selective and nonselective cyclooxygenase-2 inhibitors) and bisphosphonates (e.g., etidronate). Antiinflammatories are believed to inhibit the differentiation of primitive mesenchymal cells into osteogenic cells, whereas bisphosphonates prevent the calcification and maturation of existing osteoid. Indomethacin and rofecoxib have both been evaluated post-SCI and demonstrated to be effective for primary prevention of HO when given early after injury.13,14 Two bisphosphonates, etidronate and pamidronate, have been studied for the treatment of diagnosed HO post-SCI. Bisphosphonates inhibit the transformation of amorphous calcium phosphate into crystalline hydroxyapatite. Etidronate can inhibit progression of HO when given early, meaning a positive bone scan in the setting of negative radiographs.15,16 One study also suggests that pamidronate can halt the progression of HO following surgical resection.17 Surgery is sometimes necessary to regain range of motion, but recurrence is common, especially if resection is done during the inflammatory phase of active ossification. The full impact of SCI on voiding and the genitourinary system is underappreciated by many clinicians. Failure to address these issues can lead to significant social isolation as well as patient morbidity and mortality. In the past, renal failure was the leading cause of death following SCI.18 Fortunately, this is no longer the case, due to improved awareness, management, and surveillance of neurogenic bladder dysfunction following SCI. Today there are practice guidelines and systematic evidence-based reviews [Spinal Cord Injury Rehabilitation Evidence (SCIRE)] to guide clinical management.18,19 Bladder dysfunction after SCI can be classified as either a lower motor neuron (LMN) or an upper motor neuron (UMN) syndrome.20 LMN syndrome occurs following injury to either the conus medullaris or cauda equina. The accompanying injury to sacral (S2–4) motor neurons or their associated axons compromises motor output to the bladder, resulting in decreased or absent detrusor contractility (flaccidity). Clinical manifestations of this include urinary retention or incomplete emptying postvoid or both. UMN syndrome occurs with SCIs cephalad to the conus medullaris and is characterized by disruption of the descending spinal pathways and a loss of cortical inhibition over reflexive voiding. Immediately after SCI, the distinction between LMN and UMN syndromes can be clouded by the presence of spinal shock because the transient loss or depression of neural activity below an acute spinal cord lesion leads to initial bladder flaccidity. The majority of SCIs will exhibit UMN dysfunction due to the anatomical location of the lesion. Individuals with complete SCIs are expected to have involuntary, reflexive emptying with filling of the bladder. In comparison, many persons with incomplete SCI will have detrusor disinhibition (hyperreflexia) and urge incontinence. Communication between the pontine (brain stem) micturition center and the sacral micturition center is also disrupted or compromised. This leads to poor coordination between reflexive detrusor contractions and accompanying brain stem–mediated events, such as relaxation of the bladder neck, internal sphincter, and external sphincter. The terms used to describe this discoordination are bladder–sphincter or detrusor–sphincter dyssynergia (DSD). The net result is that the bladder reflexively contracts against the relative outlet obstruction of a closed bladder neck, internal sphincter, or external sphincter. In turn, this leads to elevated bladder pressures with micturition. Over time, elevated detrusor pressures can predispose to vesicoureteral reflux, hydronephrosis, recurrent pyelonephritis, and progressive deterioration in renal function. When reflexive voiding returns post– spinal shock, urodynamic studies should be performed to exclude occult DSD. If reflex voiding fails to return by 6 months post-SCI in a patient expected to have a UMN syndrome, urodynamic testing should also be performed. Urodynamic testing can include the following components: cystometrography, electromyography, urethral pressure profiling, and fluoroscopy. Cystometrography provides information about pressure–volume relationships in the bladder. Electromyography, using needle or surface electrodes, clarifies the function of the external sphincter and its coordination with the detrusor. Urethral pressure profiling gives information about the resistance to outflow. Fluoroscopy allows actual visualization of the bladder during voiding. An elevated voiding pressure in the face of increased sphincter electromyography activity during a detrusor contraction is diagnostic of DSD. DSD requires intervention to prevent long-term complications like vesicoureteral reflux. Goals of management focus on achieving adequate drainage, low-pressure urine storage, and low-pressure voiding. After any therapeutic intervention, follow-up testing should be performed to confirm that bladder pressures have been effectively lowered. To lower bladder pressures, reflexive contractions can be suppressed with anticholinergic agents, such as oxybutynin chloride and tolterodine tartrate. If needed, anticholinergic suppression can be augmented further with a tricyclic antidepressant. Another option is the intramuscular injection of botulinum A toxin into the detrusor body. Botulinum A toxin paralyzes muscle by blocking the release of acetylcholine at the neuromuscular junction. Disadvantages of botulinum A include expense and the fact that it has to be periodically repeated (approximately every 3 to 6 months). Following the pharmacological inhibition of detrusor contractions, bladder emptying can be safely accomplished with intermittent catheterization. Many patients with motor levels of C7 and below can be taught to perform self-catheterization. If conservative management with medications fails to work, bladder augmentation can facilitate low-pressure storage in small, noncompliant bladders. Bladder augmentation procedures with urinary diversion can also facilitate intermittent catheterization via an easier to reach abdominal stoma. In men, an alternative approach is the use of α-blockers (i.e., prazosin, terazosin, doxazosin, tamsulosin) to reduce resistance to outflow at the α-adrenergically innervated bladder neck and internal sphincter. This can lower peak bladder pressures during contractions and allow the safe use of a condom (external) catheter and collection bag attached to the leg. Transurethral and transperineal injections of botulinum A toxin have also been to used to treat dyssynergia by lowering resistance to urine outflow. Outlet obstruction can also be reduced by transurethral sphincterotomy or placement of a urethral stent. Afterward, reflexive voiding can then be managed by wearing a condom (external) catheter. For SCI patients without significant dyssynergia but with detrusor hyperreflexia, urge incontinence has been traditionally treated with systemic anticholinergic agents. Local pharmacological interventions include the intravesicular administration of capsaicin and botulinum toxin A. Capsaicin desensitizes afferent c-fibers through the depletion of substance P. In chronic SCI, the c-fibers are thought to mediate the afferent limb of the micturition reflex. Another approach to the management of UMN bladder dysfunction is the use of electrical stimulation. First, a posterior rhizotomy of the sacral nerve roots is performed to prevent reflex incontinence. Electrodes are then attached to the anterior nerve roots. Electrical stimulation of these roots causes simultaneous contraction of the detrusor and sphincters. Because the striated-muscle external sphincter fatigues before the smooth-muscle detrusor, voiding occurs in short spurts when the sphincter intermittently relaxes. LMN bladder syndrome is associated with conus medullaris and cauda equina injuries. The anatomical location of the lesion interrupts the sacral reflex arc, which consists of afferent input from the detrusor, the sacral micturition center (S2–4), and efferent input to the detrusor. The end result is detrusor areflexia (hypocontractility). Other clinical findings that accompany detrusor areflexia include saddle anesthesia, reduced anal sphincter tone, loss of voluntary sphincter control, and permanent absence of the bulbocavernosus reflex. Long-term bladder management of this patient population is straightforward and ideally consists of intermittent, clean self-catheterization timed to regularly empty the bladder and prevent bladder overdistention. If intermittent catheterization is not possible, adequate drainage can be achieved with a chronic, indwelling catheter. Recurrent urinary tract infections (UTIs) are common after SCI; however, colonization with bacteria should be expected. The presence of bacteria in the urine therefore does not warrant treatment unless the patient is clinically symptomatic (e.g., febrile) or there is laboratory evidence of tissue invasion, such as significant pyuria. The cornerstones of preventing UTIs are clean technique and regular, complete bladder drainage, because residual urine is an ideal medium for bacterial overgrowth. In addition, recurrent UTIs can be a manifestation of underlying pathology, such as kidney or bladder stones, poor hygiene, and detrusor– sphincter dyssynergia with outlet obstruction. Evaluation should therefore exclude the possibility of underlying causes. Prophylactic antibiotics should be avoided because they only serve to promote the emergence of drug-resistant organisms. Patients with SCI are also at increased risk for renal and bladder calculi secondary to factors like hypercalciuria, recurrent UTIs, and indwelling catheters. Stones in the urinarytract can present with increased lower limb spasticity, recurrent UTIs, or refractory autonomic dysreflexia. Calcified stones can often be visualized with abdominal plain films, but ultrasonography remains the gold standard for diagnosis. Bladder calculi can also be directly visualized with endoscopy. In susceptible patients, over-distention can lead to the potentially life-threatening condition of AD. To prevent this condition, most patients are advised to perform self-catheterization every 4 to 6 hours. The goal is to keep catheterization volumes less than ∼ 500 mL in men and less than 400 mL in women to minimize the risk of UTIs and other complications. Long-term follow-up is essential to maintain health and prevent complications. Although no studies have been done on the optimum frequency of follow-up evaluations, many medical centers evaluate upper and lower tract functioning on an annual basis. To guide clinicians, the American Paraplegia Society (APS) published guidelines for the urological evaluation of patients with SCI.19 Annual follow-up is recommended for the first 5 to 10 years after injury. If the patient continues to do well, the follow-up interval can be reduced to every other year. Serum creatinine should be evaluated initially and then every 1 to 2 years. The upper (kidneys, ureters) and lower (bladder, urethra) urinary tracts should also be assessed initially and then annually for the first 5 to 10 years and afterward every other year. Options for upper urinary tract evaluation include nuclear renal scans, renal ultrasound, intravenous pyelography, and high-resolution computed tomography (CT). The nuclear renal scan is probably the most effective test with the fewest adverse effects for monitoring renal function. A decrease of more than 20% in renal plasma flow warrants further investigation. The lower urinary tract can be assessed with cystoscopy, and annual cystoscopy is recommended in those with an indwelling suprapubic or urethral catheter due to the risk of squamous cell carcinoma. Urodynamics should be performed at the same intervals as upper and lower urinary tract screening. Following SCI, alterations in bowel function are a significant source of morbidity and an impediment to quality of life. Neurogenic bowel dysfunction impacts the majority of individuals with SCI, and one survey of ∼ 1300 patients found that only 1.5% reported having no bowel problems, with most patients reporting at least two significant gastrointestinal complications.21 Impaired abdominal and pelvic sensation, decreased gastrointestinal mobility, and compromised autonomic control of the alimentary tract all contribute to observed problems. The specific characteristics of neurogenic bowel dysfunction depend on the anatomical location of the injury. Injury cephalad to the conus medularis leads to a UMN bowel syndrome, whereas injury to the conus medullaris or cauda equina produces an LMN bowel syndrome. UMN and LMN syndromes require distinct management strategies, and clinicians should have a basic understanding of the underlying pathophysiology. Both UMN and LMN syndromes can be associated with prolonged gastrointestinal transit times, constipation or impaction, and incontinence. The management strategies, however, differ significantly. The UMN bowel syndrome is characterized by preserved reflexive contractions of the gut (peristalsis) allowing for continued stool propulsion; however, loss of cortical control impairs or prevents volitional defecation. This is due to the inability to voluntarily contract and relax striated pelvic floor muscles, including the external rectal sphincter, which in turn leads to prolonged constipation followed by episodes of bowel incontinence in the absence of intervention. Conversely, for LMN bowel syndrome, or “areflexic bowel disorder,” the accompanying injury at the level of the conus medullaris or cauda equina abolishes autonomic and somatic reflex arcs that receive their innervation from sacral segments. The absence of reflexive spinal–colonic connections to the descending colon and rectum leads to flaccidity and compromised stool propulsion and expulsion. Sphincter tone is also diminished or absent. On examination, sacral reflexes (e.g., bulbocavernosus, anal wink) are often absent. An effective bowel program should promote effective gastrointestinal transport, achieve regular and complete rectal evacuation, eliminate incontinence, avoid anorectal injury, and occur within a reasonable time frame in a safe, comfortable setting. This typically means employing a multifaceted approach to trigger a bowel movement at the desired time and avoid incontinence in between. Timing and a consistent schedule are the cornerstones of a bowel program, and it should be performed at the same time, typically in the morning or evening depending on individual preference. Common frequencies of bowel programs include daily or every other day, and decisions regarding the optimal frequency for an individual can be guided by preinjury bowel habits, patient lifestyle, and individual response. Typical treatment algorithms incorporate baseline recommendations for adequate fiber (15 to 30 g daily) and fluid intake (1.5 to 2 L of clear fluid daily) to promote ideal stool consistency and regularity. A primary function of the colon is water absorption and without adequate fluid intake, increased colon transit times can predispose to small hard stools and impaction. Fiber is an effective bulking agent that promotes water retention and improves peristalsis by maintaining ideal stool girth and consistency. Existing guidelines suggest starting with at least 15 mg daily.22 Thereafter, fiber intake should be individualized and titrated accordingly. Stool softeners, such as docusate sodium, can also be a helpful adjunct for managing stool consistency. In addition to promoting the consistent transport of stool through the gastrointestinal tract, a neurogenic bowel program should incorporate strategies for achieving regular and predictable stool expulsion. Here the strategies differ for UMN and LMN syndromes. Individuals with UMN syndrome can take advantage of both gastrocolic and anorectal reflexes. Distention of the stomach increases peristalsis (gastrocolic reflex); therefore, bowel evacuation is best completed 20 to 30 minutes after a meal—breakfast for a morning schedule and dinner for an evening schedule. The anorectal reflex can then be triggered following a transfer to an appropriate commode or raised toilet, or alternatively lying on the left side. The anorectal reflex increases peristaltic waves in the descending colon and rectum following rectal stimulation, thereby facilitating stool expulsion. Rectal stimulation can be achieved with digital stimulation, suppository insertion, or use of a mini-enema. The gastrocolic and anorectal reflexes are absent in the setting of LMN syndrome. Due to accompanying rectal flaccidity, individuals need to be taught to perform regular manual disimpaction to achieve evacuation, prevent incontinence, and avoid long-term complications from overdistention. If an existing bowel program proves ineffective, one variable should be changed at a time and continued for three to five cycles prior to making additional changes.22 When the time between rectal stimulation and evacuation becomes excessive or an individual fails to have a bowel movement within 24 hours of a planned evacuation, one can consider a trial of a lubricant, osmotic, or stimulant laxative. Alternatively, the form of rectal stimulation can also be changed. Polyethylene glycol based bisacodyl suppositories and mini-enemas are more effective than hydrogenated vegetable oil–based bisacodyl suppositories.23,24 For refractory chronic constipation, consideration can be given to adding a prokinetic agent. Options include cisapride, metoclopramide, and neostigmine. In spite of differences in their mechanism of action, there is level 1 evidence that both cisapride and neostigmine reduce intestinal transit times and improve bowel evacuation in the SCI population.25 Unfortunately, cisapride has been withdrawn from the United States and many other markets due to side effects. Metoclopromide acts primarily on the stomach and has been shown to be effective at promoting gastric emptying.26 Each of these medications has unique pharmacokinetic and side effect profiles that must be considered prior to prescribing. Other interventions that improve bowel motility and evacuation, include increased activity levels and warm caffeinated beverages (e.g., coffee or tea). Warm tea or coffee has the additional benefit of activating the gastrocolic reflex. If conservative management fails, more invasive therapeutic options exist. Pulsed transanal irrigation involves instilling intermittent rapid pulses of warm irrigation within the rectum with an aim to break up impacted stool and stimulate peristalsis. This therapy is administered in a retrograde fashion through an enema continence catheter secured in the anorectum with an inflatable balloon. In more extreme cases, colonic irrigation has been delivered in an anterograde fashion through an ostomy created surgically with the appendix, known as an “appendecostomy.” Although currently not routine practice, some authors advocate colostomy as a safe and effective treatment for severe, chronic gastrointestinal problems and perianal pressure ulcers in persons with SCI.27 Magnetic and electrical stimulation have also been evaluated in individuals with SCI25,28 and have been shown to reduce mean colonic transit time. In summary, mitigating gastrointestinal complications after SCI requires the institution of an individually tailored bowel routine centered on diet (e.g., fiber), hydration, postinjury physiology, and administration of local and systemic drug therapies as needed. In addition, it is common for persons with motor complete injuries to develop hemorrhoids, anusitis, and rectal prolapse as they age. Treatment of these conditions is analogous to that for the able-bodied population. For refractory or more complicated situations, pulse irrigation techniques and colostomy can be considered. On the horizon are functional magnetic and electrical stimulation techniques to stimulate peristalsis and reduce colonic transit time. Chronic pain is defined as pain persisting for 6 months or more and having the potential to disrupt physical functioning beyond the parameters imposed by the SCI.29 It is a common and debilitating condition following SCI, as well as one of the most challenging medical problems associated with SCI.30 Due to differences in methodology and definitions, the reported prevalence has varied widely—from 26 to 96%.31 Chronic pain is also perceived as difficult to deal with by the person experiencing it,32 as well as by the health care providers managing it.29 Furthermore, chronic pain negatively impacts quality of life and interferes with valued life activities, such as employment, sleep, recreational and social activities, therapy, and ability to engage in household chores.33 Pain following SCI is typically divided into neuropathic and nociceptive pain.34 Nociceptive pain is the result of the normal processing of stimuli that damage or disturb normal tissues. Nociceptive pain typically occurs above the level of the spinal cord lesion, has an identifiable cause, and may result from musculoskeletal problems such as fractures or rotator cuff tears. Neuropathic pain is more complex and results from the abnormal processing of sensory input due to damage to the nervous system.35 Although neuropathic pain can be identified by site (region of sensory disturbance) and by features (sharp, shooting, electric, burning, stabbing), it is difficult to identify a specific stimulus or cause, and patients may find it difficult to describe the quality of neuropathic pain.35 Typically, neuropathic pain is present at or below the level of the spinal cord lesion and can fluctuate in intensity depending on the individual’s emotional state or level of fatigue.36 The evaluation and management of chronic pain following SCI are addressed in Chapter 17. Osteoporosis following SCI is a significant source of morbidity and mortality. When it occurs below the neurological level of injury, it is referred to as sublesional osteoporosis (SLOP). As many as 25 to 46% of individuals with chronic SCI will sustain fragility fractures,37,38 most commonly of the distal femur and proximal tibia. Fragility fractures occur with events one would not typically associate with fractures, such as transfers (torsion) or low-velocity falls (compression),38,39 and frequently result in delayed union, nonunion, malunion, or lower extremity amputation.38 Twelve to eighteen months following a motor complete SCI, bone mineral density (BMD) of the hips, distal femur, and proximal tibia decline to 28%, 37 to 43%, and 36 to 50% of age-matched peers, respectively.40 The decline of BMD in persons with motor incomplete injuries (American Spinal Injury Association Impairment Scale [AIS] C and D) is less predictable, and there is controversy regarding whether hip and knee BMD stabilizes or continues to decline with chronic injury.41–45 SLOP is unique to persons with SCI and is characterized by lower extremity bone resorption, changes in hip and knee region bone architecture, and increased risk of lower extremity fractures. Diagnosing SLOP and assessing fracture risk entail measuring BMD and reviewing risk factors. Risk factors after SCI include SCI before age 16, duration of SCI > 10 years, paraplegia (vs tetraplegia), body mass index (BMI) ≤ 19, alcohol intake > five servings per day, motor complete SCI (AIS A or B), female gender, prior history of fracture, and maternal family history of fracture.40 Dual x-ray absorptiometry (DXA) is the standard clinical tool for diagnosing SLOP and monitoring treatment effectiveness; although peripheral quantitative CT (p-QCT) is increasingly available. DXA measures areal BMD [aBMD = bone mineral content (BMC)(g)/area (cm2)], whereas p-QCT measures volumetric BMD [vBMD = BMC (g)/volume (cm3)]. BMD T-scores or Z-scores of the hip or knee regions can identify patients with SLOP based on their gender and age at scan acquisition (Table 22.1). Assessment of knee region BMD is crucial because it is the best predictor of knee region fracture risk after SCI.45,46 BMD fracture thresholds are values below which fragility fractures begin to occur, whereas fracture breakpoints are values below which the majority of fractures occur.47 Knee region aBMD and vBMD thresholds for fracture and breakpoint have been identified.46,48 Knee fracture thresholds are ≤ 0.78 g/cm2 (aBMD), < 114 mg/cm3 (vBMD-femur), and < 72 mg/cm3 (vBMD-tibia), whereas the accompanying fracture breakpoint is < 0.49 g/cm2 (aBMD). Increases in BMD are presumed to be a suitable surrogate outcome for fracture risk reduction. Identification of lifestyle behaviors and secondary metabolic causes of low or declining BMD can be accomplished via serum screening and simple questions regarding daily or weekly caffeine or alcohol intake and smoking history (Fig. 22.1). Hypothyroidism, secondary hyperparathyroidism, renal insufficiency, vitamin D deficiency, hypogonadism (men), and amenorrhea (women) are frequently identified secondary causes of decreased BMD. Smoking cessation, caffeine intake (≤ 3 servings/day) and alcohol intake (≤ 2 servings/day) are targets for behavioral interventions. Table 22.1 Definition of Sublesional Osteoporosis (SLOP)
The Management of Secondary Complications Following Spinal Cord Injury
 Autonomic Dysreflexia
Autonomic Dysreflexia
 Cardiovascular Disease
Cardiovascular Disease
 Heterotopic Ossification
Heterotopic Ossification
 Neurogenic Bladder Dysfunction
Neurogenic Bladder Dysfunction
Physiological Alterations after Spinal Cord Injury
Upper Motor Neuron Syndrome
Lower Motor Neuron Syndrome
Other Genitourinary Issues after Spinal Cord Injury
Long-Term Screening and Follow-Up
 Neurogenic Bowel Dysfunction
Neurogenic Bowel Dysfunction
UMN Bowel Syndrome
LMN Bowel Syndrome
Goals of a Bowel Program
Fluid and Fiber Intake
Additional Aspects of Bowel Management
 Pain Following Spinal Cord Injury
Pain Following Spinal Cord Injury
 Osteoporosis
Osteoporosis
Identifying Sublesional Osteoporosis and Assessing Fracture Risk
Lifestyle or Metabolic Causes of Low/Declining Bone Mineral Density Unrelated to Spinal Cord Injury
Age range | Definition |
Men ≥ 60 years or postmenopausal women | Hip or knee region T score ≤ −2.5 |
Men < 59 years or premenopausal women | Hip or knee region Z score ≤ −2.0 with ≥ 3 fracture risk factors |
Men or women age 16–90 | Prior fragility fracture and no identifiable etiology of osteoporosis other than spinal cord injury |
Note: The T score is the number of standard deviations (SDs) BMD is above or below gender-specific young adult mean peak bone mass. The Z score is the number of SDs BMD is above or below that expected for individuals of the same age and gender. Source: Craven BC, Robertson LA, McGillivray CF, Adachi JD. Detection and treatment of sublesional osteoporosis among patients with chronic spinal cord injury: proposed paradigms. Top Spinal Cord Inj Rehabil 2009;14:9 © 2009. Reprinted with permission.
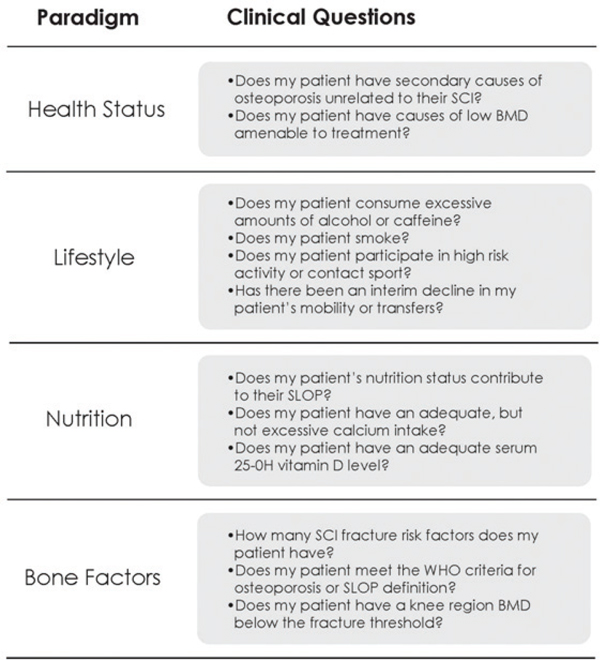
Fig. 22.1 Framework for clinical history and risk assessment for sublesional osteoporosis after spinal cord injury. (From Craven BC, Giangregorio LM, Robertson LA, Delparte JJ, Ashe MC, Eng JJ. Sublesional osteoporosis prevention, detection and treatment: a decision guide for rehabilitation clinicians treating patients with spinal cord injury. Crit Rev Phys Rehabil Med 2008;20(4):10. Reprinted with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


