CHAPTER 6 The Intervertebral Disc
Normal, Aging, and Pathologic
The intervertebral disc is a fibrocartilaginous structure whose principal function is to act as a shock absorber, transmitting compressive loads between vertebral bodies. Degeneration of the disc is associated with several clinical conditions, including herniation of the nucleus pulposus, mechanical back pain, spinal stenosis, and other spinal deformities such as scoliosis. The human intervertebral disc is considered to undergo more dramatic degenerative changes than any other musculoskeletal tissue in the body1 and to undergo these changes at an earlier age.2
Normal Disc
Disc Anatomy
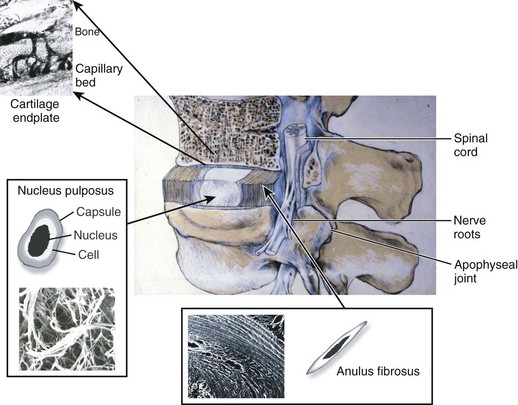
The intervertebral disc is composed of three main structures: the cartilaginous endplates, the central nucleus pulposus, and the peripherally located anulus fibrosus (Fig. 6–1).
Cartilaginous Endplates
The intervertebral disc is separated from adjacent vertebral bodies by a cartilaginous endplate superiorly and inferiorly. In humans, the endplate serves as the growth plate for the vertebral bodies, having the typical structure of an epiphyseal growth plate.3 In infancy, this growth plate is thick and occupies a substantial fraction of the disc. The endplates thin as growth progresses and eventually consist of only a 1-mm-thick, avascular layer of hyaline cartilage in adults.3 Similar to hyaline cartilage elsewhere in the body, the cartilaginous endplates are composed of rounded chondrocytes.4 Biomechanically, most compressive forces are transmitted through the superior vertebral body to the endplate, to the nucleus pulposus, and to the inferior endplate and vertebral body. The endplates and adjacent trabecular bone can undergo temporary deformation when a load is applied.
Nucleus Pulposus
The nucleus lies between adjacent endplates and forms the gel-like core of the disc. The nucleus consists of a proteoglycan and water matrix held together by an irregular network of collagen type II and elastin fibers. Proteoglycans have numerous highly anionic glycosaminoglycan (GAG) side chains (i.e., chondroitan sulfate and keratan sulfate), which allows the nucleus to imbibe water. This composition is similar to articular cartilage, and the ability of the matrix to imbibe and release water in relation to applied stresses allows the disc to cushion against compressive loads. The primary proteoglycan is aggrecan, and the high concentration of this hydrophilic molecule provides the osmotic properties needed to resist compression.5
Cells in the nucleus are initially notochordal, but their number declines after birth and they eventually become undetectable at about age 4 to 10 years.6 The nucleus is gradually replaced during growth by rounded cells resembling the chondrocytes of articular cartilage.7 These chondrocyte-like cells synthesize mostly proteoglycans and collagen type II in response to changes in hydrostatic pressure. The nucleus functions as a shock absorber, acting as a pressurized, deformable sphere that dissipates compressive forces to the anulus and the adjacent vertebral bodies. As compressive forces on the spine increase, hydrostatic pressure within the nucleus pushes outward from its center in all directions.
Anulus Fibrosus
The anulus fibrosus surrounds the nucleus and is composed of approximately 20 concentric rings (lamellae) of highly organized collagen fibers, primarily collagen type I. The collagen fibers are orientated approximately 60 degrees to the vertical axis of the spine and run parallel within each lamella but perpendicular between adjacent lamellae allowing for maximal tensile strength.8 Fibers of the outer anulus attach to the periphery of the vertebral bodies, whereas inner fibers pass from one endplate to another. Cells in the anulus are found between lamellae, arranged in parallel to the collagen fibers. Outer anulus cells are thin and elongated and phenotypically similar to fibroblasts, whereas cells of the innermost anulus are more spheroid similar to articular chondrocytes.1,9 The anulus contains the nucleus pulposus and maintains its pressurization under compressive loads. The tensile properties of the anulus allow the nucleus to recover its original shape and position when the compressive load is reduced.
Blood Supply, Nutrition, and Innervation
Blood Supply
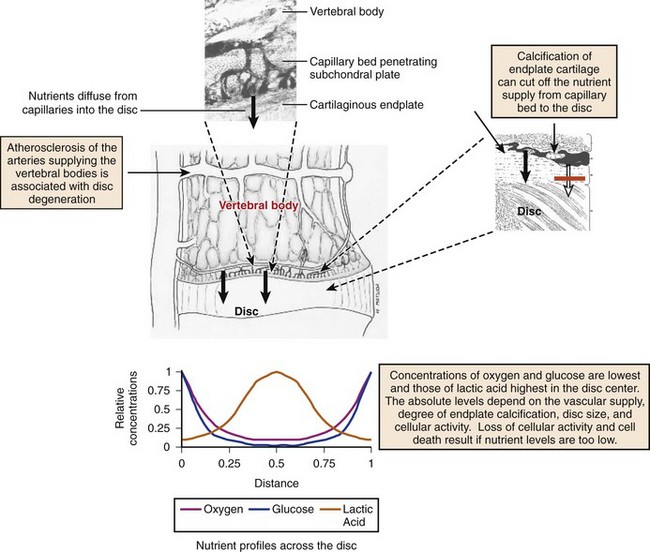
In early fetal life, vascular channels traverse the endplates, but they diminish in size starting at birth until complete disappearance by approximately 5 years of age. In adults, the blood supply of the disc arises from two capillary plexuses. One plexus penetrates 1 to 2 mm into the outer anulus, supplying only the periphery of the anulus. The other capillary plexus begins in the vertebral body and penetrates the subchondral bone (see Fig. 6–1), terminating in capillary loops at the bone-cartilage junction.10 The density of this capillary network varies in location across the endplate, being greatest in the center and lowest at the periphery. Cells in the center of the adult nucleus pulposus are 8 mm from the nearest blood source, making the disc one of the largest avascular structures in the body.
Nutrition
The limited vascularity of the intervertebral disc has important physiologic implications—mainly that nutrition depends almost entirely on diffusion (Fig. 6–2).11–13 The nutritional environment of the cells varies throughout the disc because of its size; cells in the nucleus are 6 to 8 mm from the nearest blood vessel. Small molecules necessary to maintain cellular function (i.e., glucose and oxygen) readily leave vertebral capillaries and diffuse across the thin cartilaginous endplate and the outermost layers of the anulus into the ECM. Concentration gradients of glucose, oxygen, and other nutrients and metabolites exist across the disc, regulated by the rates of nutrient supply and consumption. The low oxygen tension in the nucleus leads to anaerobic metabolism (i.e., glycolysis), resulting in a high concentration of lactic acid and a lower pH in the nucleus compared with the periphery of the disc.13 Metabolic by-products such as lactic acid are removed from the disc by diffusion in the opposite direction of nutrient entry.
Disc Composition
The function of the intervertebral disc depends greatly on the properties of the extracellular matrix (ECM). The ECM provides the biomechanical properties and acts as a filter to regulate the extracellular fluid composition and the rate at which nutrients and metabolites are exchanged. The ECM consists of a complex network of macromolecules whose composition varies in different regions of the disc (Fig. 6–3).4,14 ECM macromolecules are synthesized and maintained by a small population of cells (9000 cells/mm3 in the anulus and 5000 cells/mm3 in the nucleus) occupying less than 1% of the disc volume.4 Disc cells also produce a complex array of cytokines, growth factors, and proteases to maintain equilibrium between the rates of synthesis and degradation of ECM components.15,16
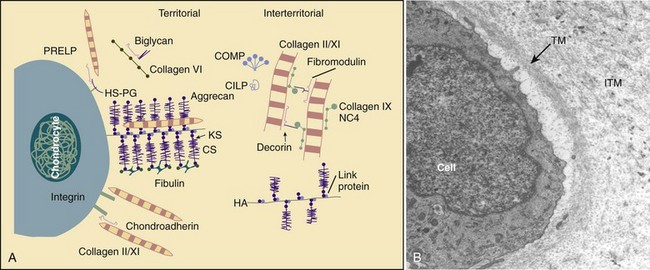
(A, Adapted from Heinegard D, Aspberg A, Morgelin M, et al: Extracellular matrix of cartilage. Section for Connective Tissue Biology, University of Lund, 2003. Available at http://www.cmb.lu.se/ctb.)
Water
The major component of the intervertebral disc is water, and its concentration is regulated by the GAG side chains of proteoglycans. The concentration of water varies with age, location within the disc, and body position.17 The nucleus pulposus is most highly hydrated, and the water concentration may be 90% in an infant, declining to approximately 80% in nondegenerated young adult discs.18 The water content of the anulus is lower than the nucleus, declining to 65% in the outer anulus in adult discs.
Water content varies with load, leading to diurnal changes in disc hydration.19 During the diurnal cycle in young, highly hydrated lumbar discs, 25% of the disc’s water can be lost and regained.20 Water is expressed from the disc during the day because of the increased forces of body weight and muscle contractions, and it is reimbibed at night when the compressive forces are removed. This diurnal cycle results in changes in disc height and affects the disc’s mechanical properties.
Macromolecules
Collagen is one major macromolecular component of the disc. The collagen content of the disc is highest in the outer anulus, and the dry weight decreases significantly in the nucleus of adult discs.21 The concentration of collagen type I is highest in the outer anulus and decreases toward the nucleus, where virtually none is present.21 Collagen type II follows the opposite gradient, with the highest concentration located in the nucleus. Along with collagen types I and II, the ECM contains many other collagens, including types III, V, VI, IX, and XI.
The other major macromolecule of the disc is aggrecan,22 which consists of a protein core with approximately 100 anionic GAG side chains. Many aggrecan molecules covalently attach to hyaluronan chains forming large aggregates. These aggregates are trapped by the surrounding collagen network, imparting a net negative charge to the ECM. The interstitial water contains an excess of cations, which is directly related to the concentration of negative charge (i.e., GAG concentration). The high concentration of cations imparts a high osmotic pressure in the nucleus, which consequently leads to imbibition of water. Changes in proteoglycan concentration and GAG concentration lead to changes in osmotic pressure, affecting the ability of the disc to maintain hydration and turgor when loaded.23
In addition to collagens and aggrecan, the disc contains lower concentrations of numerous other macromolecules,14 including elastin, the smaller proteoglycans decorin and fibromodulin, cartilage oligomeric matrix protein, and cartilage intermediate layer protein. These molecules function either structurally or biomechanically and are important for normal disc function.
Intervertebral Disc: Aging and Degeneration
Aging
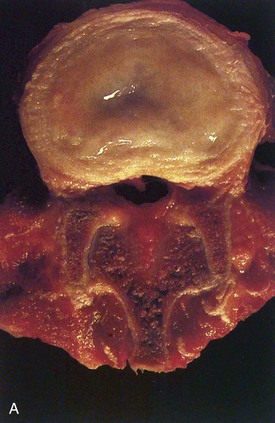
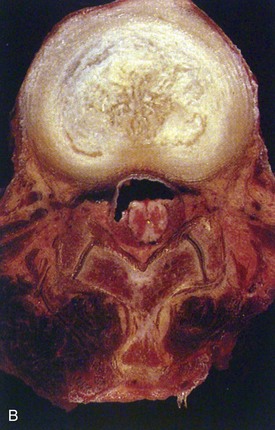
Human intervertebral discs undergo very early aging and degeneration, resulting in histomorphologic and functional changes (Fig. 6–4).24 Endplate permeability and vascular supply decrease throughout growth and aging, leading to altered metabolite transport.24 Proteoglycans begin to fragment during childhood, and the overall proteoglycan content decreases with age, especially in the nucleus. There is a corresponding increase in collagen content, with collagen type I fibers replacing collagen type II fibers in the inner anulus and nucleus. In addition, reduced matrix turnover in older discs enables collagen fibrils to become increasingly cross-linked,25 leading to retention of damaged fibers and reduced tissue strength. Synthesis of ECM components decreases steadily throughout life, and this is partly attributable to decreased cell density, although synthesis rates per cell also decrease.
In infants, the nucleus contains approximately 90% water and appears translucent.18 The disc dehydrates slowly with aging, with water content of the nucleus declining to around 80% in young adults.24 The nucleus also accumulates yellow pigmentation and becomes less distinguishable from the surrounding anulus.18,24 As the disc water content decreases, the nucleus becomes smaller and decompressed, often condensing into several fibrous lumps. Dehydration of the nucleus leads to altered biomechanical properties of the disc, forcing the anulus to act as a fibrous solid to resist compression directly. The proteoglycan content of the anulus also decreases with aging, and the anulus becomes stiffer and weaker, resisting compressive loads in a haphazard manner.
Degeneration
Intervertebral disc degeneration mimics age-related changes of the disc, but the process occurs prematurely or at an accelerated rate26,27 and usually results in symptoms. There are no widely accepted definitions of disc degeneration in the literature, reflecting the difficulty in distinguishing degeneration from the physiologic processes of growth, aging, and remodeling. More recent definitions describe degeneration as an aberrant, cell-mediated response to progressive damage, with combined structural failure and accelerated or advanced signs of aging. These proposed definitions also suggest that structurally intact discs with accelerated age-related changes be classified as early degenerative discs, whereas the term degenerative disc disease should be applied if the disc is also painful.26
Matrix Macromolecule Changes
The most physiologically important changes of disc degeneration start in the nucleus.18 Early changes include increased degradation of aggrecan and other aggregating proteoglycans coupled with an increased concentration of nonaggregating proteoglycans. The accumulation of degraded proteoglycans further impairs diffusion of nutrients and oxygen through the disc. A change in the proportions of the GAGs chondroitan sulfate, heparan sulfate, and keratan sulfate also occurs, with increasing amounts of heparan sulfate and keratan sulfate as degeneration progresses. These changes diminish the hydroscopic properties of the ECM further, resulting in decreased water content and decreased ability to imbibe water. Loss of proteoglycans and GAGs leads to decreased swelling pressure,23 loss of hydration, and loss of disc height. The changes result in altered responses to applied biomechanical loads, ultimately leading to the structural features of degeneration.
Intervertebral disc degeneration also results in disorganization and destruction of the collagen network.28 As the overall proteoglycan and water content decreases, there is a corresponding increase in collagen content. Collagen type I replaces collagen type II in the inner anulus and nucleus, and there is a tendency for collagen type I fibrils throughout the disc to become coarser. The highly organized collagen fiber arrangements of the anulus are also disrupted, and collagen and elastin networks become more haphazard. When the collagen network has been damaged, disc biomechanics are markedly altered, and the potential for structural damage increases.
The overall ECM content in the nucleus is a well-controlled equilibrium between degradative and synthetic pathways involving numerous proteins. In disc degeneration, there is an imbalance between degradative and synthetic pathways and a predominance of catabolic enzyme activity. Proteinases of the matrix metalloproteinase (MMP) and ADAMTS families cleave collagens and other macromolecules and have been implicated in the breakdown of the ECM.29 The degradative enzymes MMP-3 and MMP-13 (also known as stromelysin-1 [MMP-3] and collagenase 3 [MMP-13]) have been found at increased levels in degenerated human discs.
The regulation of MMP and ADAMTS production and ECM macromolecule production is achieved by numerous cytokines and growth factors. Of particular importance in disc ECM homeostasis are members of the interleukin (IL) family (catabolism) and transforming growth factor-β (anabolism) superfamily.30,31 Mediators of inflammation such as nitric oxide and prostaglandin E2 and the cytokines IL-1 and IL-6 are found at increased levels in degenerated discs.30–32 The synthetic capabilities of nucleus cells are unable to sustain appropriate levels of aggrecan and collagen production in the face of this increased catabolism, which contributes to further degeneration of the disc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree