Arlen D. Hanssen
The Infected Total Knee Arthroplasty: Prevention and Management
INTRODUCTION
Deep infection remains one of the most devastating and challenging complications of total knee replacement (TKR). The complexity and duration of treatment often impart significant physical, emotional, and financial costs to both the patient and treating physicians.1,2 Fortunately, the treatment of periprosthetic knee infection has become more standardized and predictable with improved clinical outcomes over the past several decades.
The overall scope of prevention, diagnosis, and treatment for the infected TKR is complex and variable. Prevention of prosthetic joint infection relies upon augmentation of the host response, optimization of the wound environment, and reduction of bacterial contamination in the preoperative, intraoperative, and postoperative time periods.3 Definitive diagnosis is often difficult and requires a heightened sense of suspicion. Treatment strategies should focus on establishing a rapid and accurate diagnosis, with clear and effective treatment algorithms to optimize long-term patient outcomes.
RISK FACTORS AND PREVENTION
Incidence rates of infection following TKR have fallen over the past few decades due to improvements in preventative efforts and surgical techniques. The most notable improvement in the reduction of postoperative deep infection occurred with the routine use of perioperative prophylactic antibiotics.4 Currently, the risk of postoperative infection following TKR is approximately 0.4% to 2%.5–7 Risk factors include patient or host variables, the surgical technique, and a multitude of aspects regarding the surgical environment and postoperative management of the TKR patient.
Host factors related to the patient’s increased susceptibility to infection should be addressed prior to the procedure; for example, those with rheumatoid arthritis or diabetes mellitus are clearly at higher risk of infection after total knee arthroplasty (TKA).7–12 Liver or kidney transplantation is an additional risk factor associated with infection in TKA.13 Human immunodeficiency virus (HIV) seropositivity14 and malignancy8 have also been shown to be independent risk factors for the development of infection after total joint arthroplasty. One must be aware of the increased risk in these immunosuppressed patient populations and take measures to minimize the potential for periprosthetic infection, such as perioperative optimization of diabetes mellitus or withholding certain deleterious rheumatoid arthritis medications.
Obesity has also been associated with increased incidence of infection.7,15 This association may be in part due to the difficulties that obesity imparts upon the surgical technique, as others have not been able to demonstrate an association of obesity with deep infection.16 Nevertheless, wound healing issues and the potential increased risk of infection in this patient population must be appreciated. Prior knee surgery is another risk factor for the development of periprosthetic infection after TKR,17 and increased rates of infection have also been demonstrated for revision knee procedures.6–8 This increased rate of infection in the revision setting may represent an expression of occult infection that goes unrecognized preoperatively and manifests itself after the revision surgery. Patients with previous infection, such as septic arthritis or adjacent osteomyelitis, are at increased risk for subsequent infection.18,19 In these patients, an acceptably low rate of deep prosthetic infection can be achieved with careful preoperative and intraoperative evaluation combined with the routine use of antibiotic-loaded bone cement (ABLC).19
Antibiotic prophylaxis represents the single most effective method of reducing infection in total joint arthroplasty.4 The optimal time to administer prophylactic antibiotics is just prior to skin incision.20 Peak serum and bone concentrations of antibiotic in TKR typically occur within 20 minutes of systemic administration and therefore, administration of prophylactic antibiotic 30 to 60 minutes prior to incision represents the optimal time of delivery.21 Although the optimal duration of postoperative prophylactic antibiotics has not been conclusively determined, it has been shown that a relatively short duration of prophylaxis postoperatively is as effective as longer periods.22 In 2004, a consensus advisory statement recommended that the first antimicrobial dose should begin within 60 minutes prior to incision and that prophylactic antibiotics should be discontinued within 24 hours of surgery.23 Although this consensus statement appears appropriate for routine primary TKR, each patient with the particular medical and surgical circumstances must be individualized on a case-by-case basis.
Curtailing risk factors associated with the surgical environment is critical in the prevention of infection after TKR. Minimization of operating room personnel24 and use of methods to reduce wound contamination such as iodophor drapes,25 clean air,7,26,27 and wearing proper surgical attire28 are all important prevention variables. Another pertinent risk factor for infection is increased duration of the surgical procedure. In a review of 6,489 TKRs, there was a significantly higher infection rate following procedures with an operative time >2.5 hours.7
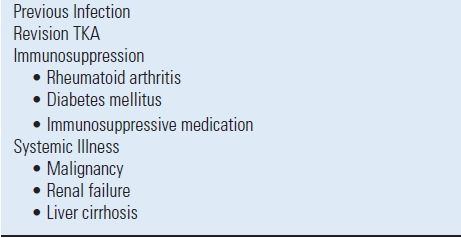
Intraoperative adjustments in high-risk patients aimed at reducing postoperative infection include the use of ABLC used for prosthesis fixation. Low-dose (<2 g of antibiotic powder per 40 g of cement) ABLC provides the necessary mechanical characteristics of cement for adequate implant fixation.29,30 Routine prophylactic use of ABLC in all patients undergoing aseptic primary TKR is currently not supported by the available clinical literature.31 There have been few prospective, randomized studies investigating the use of prophylactic low-dose ABLC in primary joint arthroplasty.32–34 With the currently available literature, we recommend low-dose prophylactic ABLC only in revision TKA and in high-risk primary TKA, such as in patients with systemic immunosuppression or a prior history of infection (Table 52.1). Further investigation, including prospective, long-term outcome studies and cost-benefit analyses, is necessary to fully elucidate whether the benefit of routine use of low-dose prophylactic ABLC outweighs the potential disadvantages that include an increase in antibiotic resistance and potential increase in allergic reactions.
TABLE 52.1 Indications for Prophylactic Low-dose Antibiotic-Loaded Cement in TKR
Hematogenous infection of TKA may occur in the early postoperative period or for many years after the arthroplasty. However, there appears to be a time-dependent wound susceptibility that is a fundamental consideration in antibiotic prophylaxis strategies to prevent bacteremia after TKR. The incidence rates of deep periprosthetic infection at the Mayo Clinic from 1969 to 1991 were analyzed.35 The highest incidence rates for all microorganisms occurred in the first three months and gradually decreased to establish a stable incidence rate between 18 and 24 months after the index arthroplasty.35 As the TKA wound and surrounding tissues heal and the inflammatory reaction subsides over the ensuing 18 to 24 months, the susceptibility of the wound and subsequent rate of infection decreases to a steady state. In general, invasive procedures that potentially cause bacteremia should simply be avoided in the first 3 to 6 months after TKR.
Antimicrobial prophylaxis for patients with a TKR who undergo an invasive surgical procedure that is a potential source of bacteremia is a source of considerable controversy. The rate of bacteremia after invasive procedures has been reported and the frequency appears to be highest with oral procedures, followed by genitourinary manipulation, and lowest in association with gastrointestinal procedures.36 In a retrospective review of 3,490 patients with TKR, 62 late infections were documented, 7 of which were strongly linked to a dental procedure.37 These authors reported that these patients had systemic risk factors for infection, such as rheumatoid arthritis or diabetes mellitus, and endured dental procedures >75 minutes in duration.
In 1997, the American Academy or Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) convened an authoritative panel of orthopaedic surgeons, dentists, and infectious disease specialists to investigate the available literature and arrive at a consensus regarding antibiotic prophylaxis prior to dental procedures. An advisory statement was released that established the first two years after arthroplasty as a specific risk factor for all patients.38 Therefore, when undergoing a high-risk dental procedure, all patients should receive antibiotic prophylaxis in the first 2 years. After that period, only the patients who are considered at high risk are given prophylaxis for the remainder of their lifetime. In the subsequent 2003 Advisory Statement, the AAOS and ADA made some modifications to the classification of patients at potential risk (Table 52.2), but made no changes in the recommended antibiotic regimens (Table 52.3).39
TABLE 52.2 Patients at Potential Increased Risk of Experiencing Hematogenous Total Joint Infection
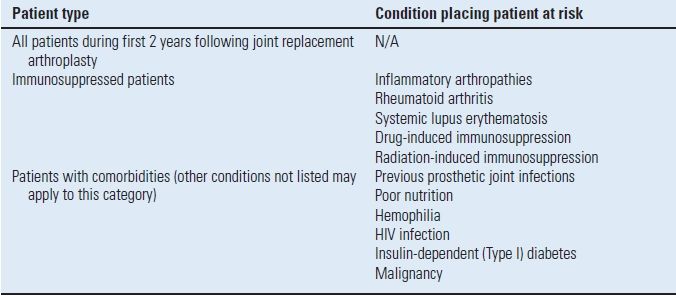
From Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 2003;134(7):895–899, with permission.
TABLE 52.3 Recommended Antibiotic Prophylaxis Regimens
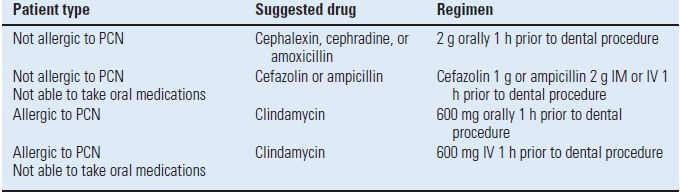
No second doses are recommended for any of the above dosing regimens.
PCN, penicillin; mg, milligram; g, gram; IM, intramuscularly; IV, intravenously.
From Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 2003;134(7):895–899, with permission.
MICROBIOLOGY
An appreciation and understanding of the microbiology are essential to the management of infected TKR. Optimal treatment outcomes are achieved by accurately identifying the involved organism and enacting medical and surgical treatment strategies that are directed accordingly. Multiple reports have documented that the most frequent organisms involved in periprosthetic infection are gram-positive, with Staphylococcus aureus (S. aureus), coagulase-negative Staphylococcus (CNS), and Streptococcus species predominating6,7,40 Specific organism distributions and patterns will vary among institutions and should be monitored closely with antibiotic resistance as part of a comprehensive infection control program.
Resistance of bacteria to specific antibiotics continues to increase via microbial genetic responses to antibiotics. Particular organisms prone to infection of total joint replacements have developed particularly troubling patterns of resistance. Methicillin-resistant S. aureus (MRSA) and methicillin-resistant CNS (MRSE) have emerged as common nosocomial pathogens often requiring complex antibiotics and subsequently associated with inferior treatment outcomes.41 In a study of 35 infected TKR, those infected with methicillin-sensitive organisms demonstrated a treatment success rate of 89% compared with only 18% treatment success in those with methicillin-resistant infections.41 Vancomycin is typically the antibiotic of choice in these methicillin-resistant infections; however, vancomycin-resistant Enterococcus species have also emerged and represent an uncommon but devastating periprosthetic pathogen.42
Periprosthetic fungal infections are rare with Candida being the predominant species.43 A review of 10 cases of candidal periprosthetic joint infection documented a successful treatment without relapse in 8 of 10 patients with appropriate antifungal therapy and a two-stage reimplantation.43 Prosthetic joint infection due to Mycobacterium tuberculosis is also rare and most often represents reactivation of prior tuberculous septic arthritis.44 To decrease the risk of reactivation of quiescent tuberculous infection, consideration should be given to perioperative antituberculous prophylaxis.44
In the presence of prosthetic implants, most bacteria elaborate a mucopolysaccharide biofilm. This represents a barrier that protects the organism from surfactants, opsonic antibodies, phagocytes, and antibiotic agents, subsequently increasing the organism’s virulence.45 These protective biofilms create an environment for persistent infection in the presence of appropriate antimicrobial therapy, especially when combined with inadequate surgical debridement. The identification and diagnosis of biofilm organisms via traditional culture methods have lacked optimal sensitivity and specificity.46 Therefore, the development of culture-independent, molecular-based methods such as polymerase chain reaction detection aims to improve our diagnostic ability and subsequent treatment of biofilm organisms. Various antibiofilm strategies directed at disruption of adherent bacteria are the focus of intense research to improve the detection of biofilm organisms and their eradication.46 Currently, rifampin is an antibiotic with good biofilm tissue penetration and has been shown to improve the treatment success when used in combination with specific other synergistic antimicrobial agents.47
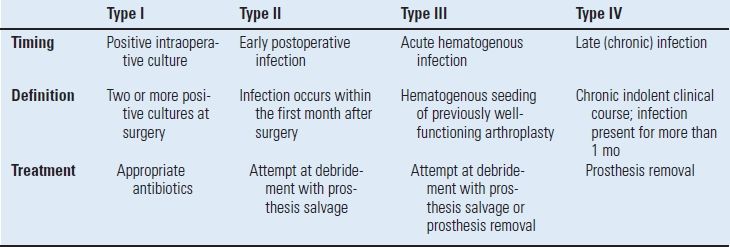
The fundamentals of diagnosis include a high index of suspicion combined with a thorough history, physical examination, plain radiographs, arthrocentesis, and hematological studies. Radionuclide studies and evaluation of surgical tissue specimens are occasionally necessary to establish the diagnosis. The timing of the clinical presentation is a critical factor in the identification and implementation of the correct treatment strategy. These various clinical presentations have been characterized and classified as a useful guide to select the most appropriate treatment option for the infected TKR (Table 52.4).48
TABLE 52.4 Classification of Deep Periprosthetic Infection
Pain is the most common presenting symptom in the patient with an infected TKR and typically occurs at rest. Persistent or progressive postoperative pain is a worrisome sign that may indicate deep infection. Persistent wound drainage is strongly suggestive of infection and should probably be treated with arthrotomy, debridement, and irrigation within the first several weeks after surgery.49 Cultures of serous wound drainage are difficult to interpret and potentially misleading and are therefore discouraged. Empiric antibiotic use for persistent wound drainage should also be avoided, as this only suppresses the clinical symptoms of infection and potentially delays diagnosis and eliminates the possibility of treatment of the infection without removal of the prosthesis.3,50–52
Diagnosis of early postoperative infection is typically confirmed by joint arthrocentesis, as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels are nonspecific in the early postoperative period. Although CRP levels typically rise to higher values after TKA than after hip arthroplasty, CRP levels after both TKA and THA peak on postoperative day number two and decrease to preoperative baseline levels to as early as 1 week but typically at 14 to 21 days postoperatively.53–55 In the early postoperative period, assertive management of delayed wound healing or marginal skin necrosis by debridement and primary wound closure is preferable to empiric antibiotic treatment, prolonged observation, and eventual development of deep infection.56
An acute hematogenous infection typically presents with sudden onset of pain or stiffness in a previously well-functioning arthroplasty.57 Specific risk factors for a hematogenous infection, such as a remote source of infection or recent invasive procedure causing a significant bacteremia, should be identified.58 The severity of symptoms with pain, effusion, and restricted range of knee motion in this setting facilitates rapid diagnosis. Although the ESR and CRP are typically elevated in these patients, the cornerstone of diagnosis is arthrocentesis with evaluation by gram stain, quantitative leukocyte count, and culture for aerobic and anaerobic bacteria. Empiric antibiotics for the unexplained painful TKR, without attempt at definitive diagnosis are unfortunately quite commonly practiced and this approach only complicates subsequent efforts to diagnose deep infection.
The vast majority of patients with an infected TKA are diagnosed in the subacute or chronic setting. Historical factors such as persistent pain since the arthroplasty, prolonged postoperative wound drainage, antibiotic treatment for difficulties with primary wound healing, and knee stiffness despite extensive rehabilitation efforts may be indicative of deep infection. Sequential comparison of plain radiographs may reveal progressive radiolucencies (Fig. 52-1), focal osteopenia or osteolysis of subchondral bone, and periosteal new bone formation.59 Additional studies should include a peripheral white blood cell count, CRP, ESR, and aspiration of the affected TKR.
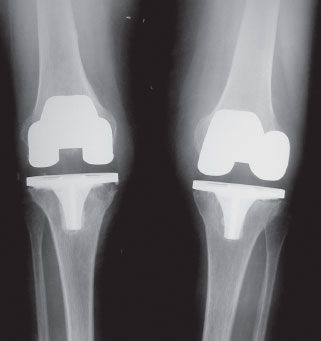
FIGURE 52-1. AP radiograph of a patient with bilateral chronically infected TKRs. Radiolucencies at the bone-cement interface, typically late findings in periprosthetic infection, can be seen inferior to the tibial components.
Arthrocentesis is considered an essential element of the workup and evaluation of a suspected deep periprosthetic infection.60–63 A synovial fluid leukocyte differential of >65% neutrophils or a leukocyte count of >1.7 × 103/µL is a sensitive and specific test for the diagnosis of deep periprosthetic infection in patients without underlying inflammatory disease.63 Patients should be off antibiotics several weeks prior to aspiration as this oversight frequently accounts for the inability to isolate organisms. In one series of 69 knees, the preoperative aspiration had a sensitivity of 55%, specificity of 96%, and accuracy of 84%.62 It is important to note that 12 of the infected knees were on antibiotics at the time ofaspiration and 7 (58%) of these knee cultures had no growth. After antibiotics were discontinued, four knees were reaspirated and all had a positive culture. The overall sensitivity improved to 75%, specificity to 96%, and accuracy to 90%.62 Isolation of microorganism(s) from the aspirate also helps direct the initial choice of antibiotics following removal of the infected prosthesis.
The use of radioisotope scans to facilitate the diagnosis of the chronically infected knee prosthesis is occasionally useful.64 A screening technetium-99 study may be helpful when the study demonstrates the absence of tracer uptake, as this excludes the possibility of deep infection. Indium-111 leukocyte scanning appears to be more accurate (78%) than technetium-99 diphosphonate bone scanning alone (74%); however, the indium-111 leukocyte scanning combined with technetium-99 sulfur colloid scintigraphy provides an improved accuracy of 88% to 95%.64
Despite all reasonable efforts to diagnose infection preoperatively, intraoperative evaluation of surgical tissue may be necessary to confirm the diagnosis in difficult cases. The Gram stain is notoriously unreliable with a high percentage of false-negative results and an extremely low sensitivity.65,66 Intraoperative frozen section testing to detect infection is widely used, yet the published results of this technique are variable.67–71 This variability is likely accounted for by differences in technique, sampling errors during retrieval of tissue samples, pathologist experience, and the definition used to declare infection. The positive predictive value of frozen sections is significantly better (p < 0.05) when the index was increased from five to ten polymorphonuclear leukocytes per high-power field.69 Analysis of intraoperative frozen section is a reasonable and reliable method when an accomplished and experienced pathologist evaluates appropriate tissue samples.
The surgeon’s intraoperative assessment is also helpful during revision surgery and if infection is suspected, one should obtain multiple tissue specimens for culture and sensitivity testing and await the results. A careful review of the history, ESR and CRP, combined with the results of cultures and the final interpretation by the pathologist will often allow the determination of whether infection is present. Our current definition of prosthetic infection includes a combination of clinical signs and symptoms, histological analysis of tissue, and culture results. The diagnosis of definite infection is made if evaluation of the knee establishes at least one of the following criteria: (a) two or more cultures obtained by aspiration or deep tissue specimens obtained at surgery yield the same organism, (b) histopathologic evaluation of intra-articular tissue reveals acute inflammation, (c) gross purulence is observed at the time of surgery, or (d) an actively draining sinus tract is observed.72
TREATMENT
Once the diagnosis has been established, the variables that must be considered before initiating treatment include (a) whether the infection is superficial or deep, (b) the duration of time between the TKR and diagnosis of infection, (c) host factors that may adversely affect the treatment of the infection, (d) the condition of the soft tissue envelope surrounding the knee and specifically the integrity of the extensor mechanism, (e) whether the implant is loose or well fixed, (f) the pathogen(s) responsible for the infection, (g) the physician’s ability to provide the proper level of care required, and (h) careful assessment of the patient’s expectations and functional requirements.
Treatment goals of the infected TKA include eradication of infection, alleviation of pain, and maintenance of a functional extremity. The six basic treatment options include (a) antibiotic suppression, (b) open debridement, (c) reimplantation of another prosthesis (d) arthrodesis, (e) resection arthroplasty, and (f) amputation. With the exception of chronic antibiotic suppression, which does not eliminate infection, the cornerstone principles of these treatment options include thorough surgical debridement combined with the appropriate use of antibiotics and optimization of the host response. Upon diagnosis of infection, the first step is determining whether the prosthesis can be retained or if removal is required to treat the infection.
Treatment Methods with Component Retention
Antibiotic Suppression Antibiotic treatment alone will not eliminate deep periprosthetic infection but can be used as suppressive treatment when the following criteria are met: (a) prosthesis removal is not feasible due to the patient’s medical condition or other reasons, (b) the microorganism has low virulence, (c) the microorganism is susceptible to an oral antibiotic, (d) the antibiotic can be tolerated without serious toxicity, and (e) the prosthesis is not loose.73 The presence of other joint arthroplasties or a cardiac valvular prosthesis is a relatively strong contraindication to chronic antibiotic suppression as a treatment choice.
In a multicenter study, antibiotic suppression was successful in only 40 (18%) of 225 knees,74 while combining several series reveals that antibiotic suppression was successful in 62 (24%) of 261 knees.6 Use of a combined regimen of rifampin with a quinolone has been reported to be more successful than treatment with a single antibiotic.75 Despite the fact that most patients fail to meet all of these selection criteria, antibiotic suppression is commonly practiced and unfortunately prolongs the presence of infection and often complicates subsequent treatment attempts. Long-term antibiotic suppression should be rarely initiated and only considered when all the above treatment criteria are met.
Debridement with Prosthesis Retention Open debridement may be indicated for an acute infection in the early postoperative period (Type II) or for acute hematogenous infection (Type III) of a well-fixed prosthesis. Suggested criteria for this treatment technique include: (a) duration of symptoms of infection <2 weeks, (b) susceptible gram-positive organisms, (c) absence of prolonged postoperative drainage or sinus tract, and (d) no prosthetic loosening or radiographic evidence of infection.3 A relative contraindication for debridement and attempted salvage of the prosthesis is the presence of other joint replacements or a cardiac valve prosthesis.
Results of debridement are difficult to determine due to differences in microbiology and subsequent antibiotic management, variability in the time to treatment, quality of the soft tissue envelope, extent and completeness of debridement, status of implant fixation, and the criteria for success in each report. A multicenter study reported a success rate of only 19.5% with open debridement of 154 knees.74 In a recent literature review, a success rate of only 32.6% was reported in 530 infected TKRs treated with open debridement and component retention.76 Factors identified with failure of open debridement included postoperative drainage >2 weeks in duration, existence of a sinus tract at debridement, hinged prostheses, and immunocompromised hosts.76
The importance of the timing of the debridement in relationship to the onset of symptoms or the period of time elapsed since the insertion of the prosthesis cannot be overemphasized.3,48,50,77,78 In a study of 24 infected TKRs treated with open debridement and component retention, successful treatment was demonstrated in 100% in the early postoperative infection group (Type II) and 71% of the acute hematogenous group with <30-day duration of symptoms.77 These authors emphasized strict selection criteria for component retention, with patients demonstrating evidence of infection for <30 days.77 Debridement with prosthesis retention should not be attempted in patients with chronic infections.48,79
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








