Techniques to Manage Infection around Total Hip Arthroplasty and Antibiotic-Loaded Spacers for Infection
Michael C. Parry
Clive P. Duncan
Bassam A. Masri
INTRODUCTION
Total hip arthroplasty has developed into one of the most successful interventions for the treatment of disabling degenerative disease of the hip (1). However, while uncommon, periprosthetic joint infection (PJI) of the hip remains a devastating complication for the patient (2) and continues to challenge surgeons involved with their treatment. PJIs of the hip place a significant economic and logistic burden on the institutions involved with their care (3,4), and with the demand for total hip
arthroplasty expected to increase in the next two decades (5), it is likely that this burden will also increase.
arthroplasty expected to increase in the next two decades (5), it is likely that this burden will also increase.
The treatment of PJI of the hip remains an area of controversy. It is widely accepted among the North American orthopedic community that the two-stage approach, in which the removal of infected material and components and reinsertion of revision components are separated by an interval period with either no components or antibiotic-loaded components in situ, is the gold standard treatment for PJI of the hip (6,7). However, this strategy may be associated with increased morbidity and mortality and certainly carries a cost implication above that of a one-stage approach unless the latter fails (8,9,10,11). Proponents of the single-stage approach argue that the successful outcome following revision for PJI, as measured by eradication of infection, is determined by the quality of the initial debridement, and as long as strict protocols are adopted, reinfection rates are at worst comparable to those seen with a two-stage approach (12,13). In spite of these findings, a number of systematic reviews and meta-analyses have failed to identify a superior approach to the management of PJI of the hip (14,15,16,17,18), which may, in part, reflect the multifactorial nature of periprosthetic infection as well as the heterogeneous populations included in the included studies.
When considering the two-stage approach to the management of PJI of the hip, consideration must be given to whether a spacer is used between stages and, if so, to the nature of the spacer utilized. Spacers are formed from antibiotic-loaded cement, the purpose of which is to deliver high local concentrations of antibiotic through elution from the cement. These spacers can be articulating or nonarticulating and custom-made or preformed. Nonarticulating spacers are not designed for weight bearing or for keeping the limb out to length and so inevitably result in some contraction of the soft tissues of the hip during the interval period. However, they do offer a reduced risk of complications including spacer dislocation and fracture. In contrast, articulating spacers are designed to keep the limb out to length, and some may allow some weight bearing and thus offer improved function of the hip and preservation of the soft tissue envelope, but do carry an increased risk of complications (19).
The purpose of this chapter, therefore, is to report the techniques available for interval spacers in the two-stage treatment of PJI of the hip, addressing both the static and articulating varieties.
INDICATIONS
The successful treatment of PJI of the hip by two-stage revision is reliant on the eradication of infection during the interval period before reimplantation. This is dependent on a thorough debridement at the time of the first stage, designed to remove all foreign, infected, and devitalized material. In conjunction, an identification of the infecting pathogen, including its antibiotic sensitivities, is vital to direct antimicrobial therapy between stages and thus improved outcomes.
Antibiotic spacers are indicated for the treatment of chronic infections, infections due to resistant or fungal organisms, and in patients in whom host factors are likely to result in failure with a one-stage approach (20,21,22). While the time between onset of symptoms and surgical intervention often guides the choice between component retention and two-stage revision, the exact duration of symptoms permitting component retention remains controversial. Certainly, the longer symptoms have been present, the more likely biofilm formation, osteomyelitis, and sinus formation are to be established, associated with a higher risk of failure with more conservative revision options (23,24). As the first stage of a two-stage approach involves removal of the prosthesis, this offers the surgeon the opportunity to access bone and soft tissues adjacent to the components as well as reduces the bacterial burden in association with the prosthesis. The choice between an articulating or static spacer at this stage is largely at the discretion of the operating surgeon though a static spacer may be more appropriate in cases of severe bone loss and for surgeons with limited experience with the subtleties of articulated spacers and their limitations.
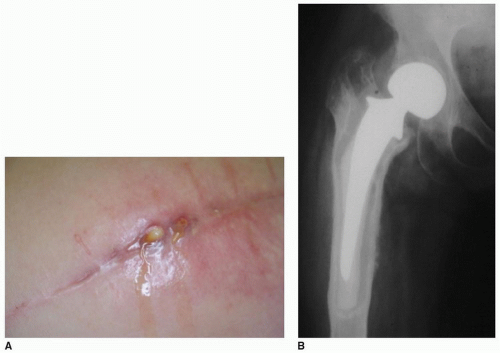
The accurate diagnosis of PJI of the hip remains a controversial and evolving subject. It is reliant on not only an accurate history and examination but also investigation of radiographs, blood, and synovial fluid. All hip arthroplasties presenting with ongoing pain should be considered infected until proven otherwise, especially when the onset of symptoms correlates with concurrent illness or surgical intervention. Cutaneous manifestations of PJI of the hip are often absent though the presence of a sinus is invariably indicative of deep infection (25) (Fig. 30-1). Systemic inflammatory
markers including white cell count (WCC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) are sensitive though nonspecific markers of infection and serve as first-line screening investigations (26). The diagnosis of PJI has been defined by the Musculoskeletal Infection Society (MSIS) on the basis of one of two major criteria (a sinus tract communicating with the prosthesis or a pathogen isolated by culture from tissue or fluid samples) or four of six minor criteria (elevated CRP and ESR; elevated synovial leukocyte count; elevated synovial neutrophil percentage; presence of purulence in the affected joint; pathogen isolated from one sample of tissue or fluid from the affected joint; greater than 5 neutrophils per high-power field at ×400 magnification on frozen section analysis of periprosthetic soft tissue) (27). While these guidelines have a role in the accurate diagnosis of PJI, there will always be cases that defy these criteria in which clinical assessment remains the gold standard for diagnosis.
markers including white cell count (WCC), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) are sensitive though nonspecific markers of infection and serve as first-line screening investigations (26). The diagnosis of PJI has been defined by the Musculoskeletal Infection Society (MSIS) on the basis of one of two major criteria (a sinus tract communicating with the prosthesis or a pathogen isolated by culture from tissue or fluid samples) or four of six minor criteria (elevated CRP and ESR; elevated synovial leukocyte count; elevated synovial neutrophil percentage; presence of purulence in the affected joint; pathogen isolated from one sample of tissue or fluid from the affected joint; greater than 5 neutrophils per high-power field at ×400 magnification on frozen section analysis of periprosthetic soft tissue) (27). While these guidelines have a role in the accurate diagnosis of PJI, there will always be cases that defy these criteria in which clinical assessment remains the gold standard for diagnosis.
The rational for a two-stage approach is the assumption that even an aggressive debridement cannot reliably produce a sterile field. Much like the use of chemotherapy following tumor resection, antibiotics eluted from the cement spacer and administered systemically will eradicate any organisms remaining at the site of infection, and systemic antibiotics will further kill any residual organisms. Therefore, for this to be successful, a pathogen and its antibiotic sensitivities should be identified. When suspected, a preoperative aspiration from the hip should be performed under radiologic guidance (28). Fluid obtained under aseptic techniques must be analyzed for cell count and differential and also cultured to confirm the species and sensitivities of the infecting organism. Prolonged culture up to 14 days is often required and should be stipulated at the time of retrieval, as many pathogens implicated in PJI are slow growing in routine culture environments. The identified pathogen can be used to guide the choice of antibiotic added to the cement at the time of first-stage revision and the systemic antibiotic used during the interval period (29).
CONTRAINDICATIONS
A patient-centered approach should be applied to the treatment of PJI of the hip. Two-stage revision presents a significant physiologic insult to the patient who is often compromised on the basis of comorbidities including diabetes, immunosuppression, cardiac, respiratory, or renal impairment. While the two-stage approach remains the appropriate intervention for the treatment of PJI of the hip in the majority of cases, it carries a high cost in terms of mortality and morbidity (8). In such patients in whom aggressive, staged surgical intervention is associated with an unacceptable predicted risk of mortality, an alternative intervention, including chronic antibiotic suppression, may be a more appropriate treatment strategy (30,31).
PREPARATION
Having completed preoperative investigations and following discussion with the patient of the protracted nature of the two-stage treatment approach, preparation for the first stage begins with preoperative assessment of up-to-date radiographs. Radiographs should be assessed for the degree of bone loss on both the femoral and acetabular sides as well as evidence of established osteomyelitis. The presence of cement and the volume of proximal femur filled by prosthesis, cement, and plug should be established as all this material will need to be removed at the first stage (32). Knowledge of the infected prosthesis, including component sizes, should be established preoperatively and often necessitates communication with the index hospital to trace the original implant labels, which are located in the medical record. Ideally, the operative report from the index procedure should be obtained as this will aid in planning the approach for the first stage of the revision. Specialized implant extraction instruments are often required in readiness for the first-stage procedure. In the case of well-fixed fully coated uncemented prostheses, or cemented prostheses without evidence of lucency at the cementbone interface, an extended trochanteric osteotomy may be required and should be anticipated prior to the surgery date. On the acetabular side, significant bone loss, particularly of the anterior and posterior column or migration of the acetabular component medial to Kohler’s line, should be noted. In such circumstances, a nonarticulating spacer may be more appropriate in an attempt to preserve as much residual pelvic bone stock ahead of the second-stage reimplantation.
As often as possible, antibiotics should be withheld in the period leading up to the first-stage procedure, especially where the diagnosis is in doubt and intraoperative frozen section is anticipated. An integrated management strategy encompassing surgeon, pathologist, and infectious diseases specialist, preferably with an interest in PJIs, is mandatory to the successful treatment of PJI of the hip, and involvement early in the treatment is recommended.
TECHNIQUE
The principle aim of the first stage is the removal of all infected and nonviable material, including prostheses, cement, soft tissue, and bone. This is reliant on adequate exposure. The previous incision should be extended to allow adequate exposure, excising any sinuses within the incision and excising the tract down to the prosthesis. Regardless of surgical approach, adequate exposure of the prosthesis and periarticular region must be achieved to allow a complete debridement. On entering the hip joint, samples of fluid and tissue from both the acetabular and femoral region, as well as any suspicious material, should be sent for microbiologic assessment (33). It is at the surgeons’ discretion whether to expose the acetabulum first, following removal of the femoral head if possible, or to address the femoral component first. In the case of a cemented femoral stem, it is our preference to remove the stem first, remove the acetabular component next, and finally remove the femoral cement last. This maintains strength in the femur to allow safe retraction while working on the acetabular side and it reduces blood loss. Exposure often requires removal of much of the thickened capsular tissue to allow dislocation. Retraction of the femur then allows exposure of the remainder of the capsule and synovium, which can then be debrided. Debridement of the synovium is vital to the identification of the implant-bone interface, which will aid in their removal. Extraction of the femoral component will often require clearance of the shoulder of the prosthesis, which often necessitates removal of a portion of an overhanging greater trochanter using a high-powered burr to prevent fracture on extraction. Shorter, proximally coated stems can often be removed from within the femoral canal using a combination of flexible osteotomes, saws, and burrs, without a femoral osteotomy (Video 30-1). On the other hand, well-fixed fully coated stems, fluted tapered titanium stems, and well-fixed cement mantles necessitate an extended trochanteric osteotomy. Whichever is used, preservation of the femoral bone stock, so long as it does not jeopardize the quality of the debridement, is paramount for reimplantation at the second stage. Removal of a well-fixed cement mantle can present a challenge. As for the removal of an uncemented prosthesis, this can be achieved
from within the femoral canal using a combination of osteotomes, high-speed burrs, and ultrasonic cement removal tools, depending on the surgeon’s preference. In the case of a long cement mantle or a low placed cement plug, an extended trochanteric osteotomy should be considered.
from within the femoral canal using a combination of osteotomes, high-speed burrs, and ultrasonic cement removal tools, depending on the surgeon’s preference. In the case of a long cement mantle or a low placed cement plug, an extended trochanteric osteotomy should be considered.
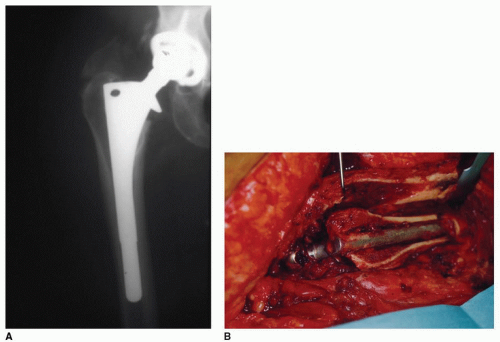
While it is beyond the scope of this chapter to describe the surgical technique of extended trochanteric osteotomy, we wish to emphasize the importance of retaining the blood supply of the osteotomy fragment, particularly when the saw blade cannot traverse the medullary canal, which contains a canal-filling cementless stem (Fig. 30-2).
Removal of the acetabular component relies on adequate exposure and should only be attempted following a thorough debridement of the periarticular capsule and synovium with adequate retraction of the femur. In the case of an uncemented component, the liner and any acetabular screws should be removed. Using a combination of size-specific curved thin blades with a diameter matched to the existing acetabular component (34), most shells can be removed with minimal violation of the host bone stock. In the case of a cemented acetabular component, a combination of reamers and osteotomes is usually sufficient. In principle, the polyethylene socket should be removed first from the cement mantle, which can then be removed from the underlying bone using osteotomes. Removal of a loose cemented socket is typically fairly simple; however, removal of a well-fixed cemented acetabular component can incur damage to the underlying bone unless great care is taken. In such cases, reaming of the polyethylene socket may be considered if it cannot be easily removed from the cement mantle using osteotomes.
Following removal of the components and any cement, a thorough debridement of the exposed bony anatomy is undertaken. The thick fibrous membrane often present at the bone-implant interface must be removed from the femoral canal and the acetabulum, which often necessitates a combination of burrs, reamers, and reverse cutting hooks. Debridement of the acetabulum is often achieved with an appropriate-sized reamer, taking into consideration any areas of bony deficiency.
At this stage, the debrided bed is irrigated with a large volume of solution. While the exact volume and nature of this irrigate remains open for debate (35,36), it is our practice to irrigate the bed with a minimum of 9 L of normal saline with additional antibiotic (Bacitracin 50,000 per liter normal saline) via low-pressure pulsed lavage.
The purpose of the cement spacer is to allow continued elution of antibiotic establishing local concentrations well above the minimum inhibitory concentration of the infecting organism. Secondary
goals are the preservation of limb length and function, preservation of the soft tissue envelope, and facilitation of re-exposure at the second stage (37).
goals are the preservation of limb length and function, preservation of the soft tissue envelope, and facilitation of re-exposure at the second stage (37).
Historically, antibiotic cement beads were often used, though these have largely been abandoned in favor of solid spacers, which offer improved soft tissue preservation and aid removal at the time of the second-stage procedure (38).
Static Cement Spacers
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree