Chapter 36 Synthetic Meniscal Substitutes and Collagen Meniscal Implantation
The structure and composition of the C-shaped fibrocartilaginous menisci enable the functional performance of the knee joint during activities of daily living. Wedged between the incongruent surfaces of the tibia and femur, the menisci help distribute loads across the articulating surfaces and, in doing so, help protect adjacent articular cartilage. The tissue contains an inhomogeneous anisotropic arrangement of collagen type I fibers combined with proteoglycans, glycoproteins, and elastin.4 The circumferentially oriented collagen fiber bundles are contiguous with the anterior and posterior horns that anchor the meniscus to the tibial plateau. These anchor sites help the tissue resist extrusive forces and allow the menisci to contribute to joint stability.24
Sometimes, the meniscus cannot withstand the mechanical burdens placed on it. Traumatic tears are most commonly observed in young, athletically active individuals, whereas degenerative meniscal tears tend to occur in patients older than 40 years. If damage occurs in a vascularized zone, the tissue can heal with a cellular fibrovascular scar similar to that which occurs in other soft tissues. However, healing is slow, with months or even years required for the scar tissue to mature to normal fibrocartilage. The mechanical function and strength of the repair tissue are unknown, so even when healing is complete, the residual strength and thus the function of the meniscus may be compromised. When meniscal injuries occur in the avascular region of the meniscus, common for degenerative tears, no reparative response is possible. Surgeries to treat damaged menisci number 850,000 per year in the United States but despite the prevalence of meniscal injuries, surprisingly few treatment options exist.28
Treatment Options
The ultimate goal of any surgical intervention is to relieve pain while restoring the function of the meniscus so that it can contribute to knee stability and protect surrounding musculoskeletal tissues from degeneration. There are four options to treat a damaged meniscus: (1) removal; (2) repair; (3) substitution (allograft or synthetic implant); and (4) scaffold implantation. The treatment used depends on the location and extent of the damage, age and activity level of the patient, and presence of concomitant injuries, such as rupture of the anterior cruciate ligament, which places increased mechanical demands on the healing tissue.14
Removal of Meniscal Tissue
The deleterious effects of total meniscectomy include pain, recurrent swelling, limited mobility and ultimately osteoarthritic degeneration.1,11,13,19 The subsequent deterioration of the joint leads to disability, the need for multiple surgeries and, often, total knee joint replacement. Although complete meniscectomy has fallen from favor, removal of a portion of the meniscus remains a commonly performed procedure. A recent cadaveric study reported a direct relationship between the amount of the posterior medial meniscus removed and the increase in articular cartilage contact stress.23 However, the minimum amount of meniscal tissue that can be removed without negatively impacting the functional performance of the joint remains unclear.
Meniscal Repair
The ability to repair a torn meniscus is dependent on the location and type of tear. Whereas tears in the vascular periphery of the meniscus have the ability to heal, most of the inner meniscal tissue is avascular and therefore has poor healing potential. There is much interest in developing methods to augment healing by using cytokines and other bioactive moieties.16,37
Early work by Arnoczky and colleagues,5 for example, evaluated the use of a fibrin clot in meniscus healing. Full-thickness lesions in the avascular portion of the medial meniscus of 12 adult dogs were filled with an exogenous fibrin clot. The defects filled with a fibrin clot healed through a proliferation of fibrous connective tissue that eventually modulated into fibrocartilaginous tissue. It was concluded that the fibrin clot acts as a chemotactic and mitogenic stimulus for reparative cells and provides a scaffold for the reparative process. Scotti and associates31 have suggested augmenting repair sites with a chondrocyte-rich solution encapsulated within an acellular fibrin glue. Harvested swine menisci were used to create a trilayered construct consisting of a layer of chondrocyte-fibrinogen solution glue sandwiched between two layers of harvested swine menisci and wrapped in acellular fibrin glue. After 4 weeks of implantation into the subcutaneous tissue of nude mice, the acellular fibrin glue exterior had been replaced with a neovascular capsule, which allowed for tissue growth between the two sections of the meniscus. Hypercellular fibrocartilaginous tissue, active remodeling of the meniscal tissue, and bonding across the interface were evident.
The effect of insulin-like growth factor-1 (IGF-1) on meniscus healing was recently assessed in a goat model.41 Full-thickness meniscal defects were created in the avascular region of the meniscus in 48 goats and bone marrow stromal cells transfected with the hIGF-1 gene were delivered to the healing meniscus using a calcium alginate gel delivery vehicle. At 16 weeks, the repaired meniscal defects were filled with white tissue similar to that in normal meniscal fibrocartilage. The repair tissue was composed of cells embedded within matrix that filled the interfibrillar spaces. Notably, the proteoglycan content in the gene-enhanced group was higher than that in the control groups.
Meniscal Substitution
Despite limitations, which include incomplete graft incorporation, difficulty in sizing, availability, immunologic rejection, disease transmission, and tissue integrity, meniscal substitution in the form of an allograft has been indicated for patients who have had a complete meniscectomy.27 Short-term studies have demonstrated improvements in symptoms (pain and swelling) following meniscus transplantation; however, the ability of a meniscus allograft transplant to provide long-term chondroprotection is unproven.
Replacing the entire meniscus with a synthetic nondegradable implant that can carry and distribute load without damaging the articular surfaces of the joint has been a long-standing goal in the field of musculoskeletal soft tissue research. However, finding the optimal combination of synthetic materials to allow for a wear-resistant functional substitute has been difficult, with the result that no synthetic implant is clinically available as yet. Nondegradable hydrogels, such as polyvinyl alcohol (PVA), have been suggested as suitable meniscal substitute materials because of their high water content, low coefficient of friction, and stability when implanted. In small animal models, PVA-based total meniscal replacements demonstrated an ability to protect cartilage and remain intact for periods of up to 12 months.22 PVA-based implants were also followed for up to 12 months in an ovine model and, at 2 months postoperatively, the implants demonstrated an ability to protect the knee joint from degeneration as compared with the meniscectomized knee.21 At 4 months, however, the tibial plateau was significantly more degenerated when compared with that of the allograft implanted group, with degenerative changes particularly evident on the peripheral region of the tibial plateau. By 12 months, radial tears in the posterior aspect of the implant were evident. These studies highlight the challenges of designing a substitute that can withstand the rigorous mechanical environment of the knee.
Scaffold Implantation
Collagen Meniscal Implant
In December 2008, the U.S. Food and Drug Administration (FDA) approved the first collagen-based meniscal implant for commercial use in the United States, known as Menaflex (ReGen Biologics, Franklin Lakes, NJ).25 Note that through its development, this was referred to as a collagen meniscal implant (CMI). When ReGen Biologics received FDA approval to market the device for sale in the United States, Menaflex became its preferred (trade) name. We will use the term collagen meniscal implant (CMI) in this chapter to refer to the Menaflex product.
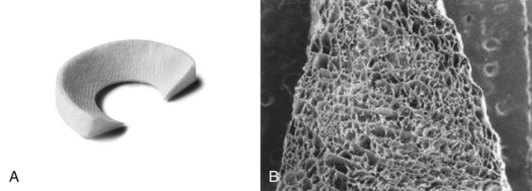
CMI is manufactured from type I collagen harvested from bovine Achilles tendon (Fig. 36-1). The harvested tendon is washed, the collagen fibers are isolated and purified using sequential chemical treatments and organic solvents, and the purified collagen fibers are swollen in the presence of equal quantities of hyaluronic acid and chondroitin sulfate. Glycosaminoglycans are added and the resulting compound is coprecipitated by the addition of ammonium hydroxide. The collagen fibers and associated extracellular components are then dehydrated and manually oriented in a mold. The resulting structure is lyophilized and sterilized by gamma irradiation. The end product is an acellular scaffold intended to support cell migration and de novo tissue growth from existing meniscal tissue, the synovium, and synovial fluid.34 The collagen meniscal implant can be trimmed to match the specific dimensions of a patient’s meniscal defect. The resulting implant has the tensile strength to support attachment of the implant to a rim of the native meniscus with sutures and immediately withstand the sheer and compression forces within the knee joint, while maintaining a porous matrix to allow tissue regeneration. The collagen meniscal implant has been studied in humans to replace partial meniscal defects of the medial and lateral meniscus. In Europe, both medial and lateral implants are available for surgical implantation, whereas in the United States, only the medial implant is currently available for use.
Pathway to Clinical Use
Although CMI has only been recently approved for use in the United States, it has been available in Europe for several years. CMI developed involved almost 2 decades of research and consequently its performance in vitro and in vivo has been studied extensively.29,30,32–34 The construct has been shown to support cellular ingrowth and encourage tissue regeneration in many small and large animal models. Stone and associates,33 for example, have evaluated CMI in a canine model. An 80% subtotal resection of the medial meniscus was created and the defect was then treated with the collagen-based scaffold. The investigators found that the implant was compatible with meniscal fibrochondrocyte ingrowth and concluded that it could induce regeneration of the meniscus in the mature dog.
Clinical Indications
CMI is indicated for use in individuals who have acute or chronic medial meniscal damage that is irreparable via suturing. A peripheral portion of the native meniscus must be intact to allow for secure attachment of the scaffold and to allow for cell ingrowth. Significant chondral damage, knee malalignment, and instability are contraindications. Concomitant ACL reconstruction or an osteotomy to correct malalignment may be done with scaffold implantation. The clinical trials that have been completed thus far have been primarily conducted on men, but gender is not an inclusion or exclusion criterion.30
Surgical Technique, Postoperative Care, and Rehabilitation
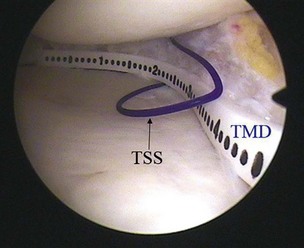
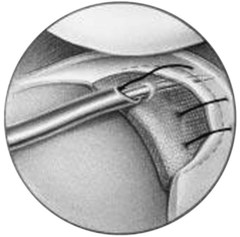
CMI is intended to be implanted arthroscopically. The medial meniscus is débrided to the vascular zone and, using a customized measuring device, an implant is chosen for the specific defect. A standard inside-out suturing technique is used to secure the implant. A posteromedial incision is used for placement of a posterior retractor. Before the implant is inserted (Fig. 36-2), a temporary suture is placed in the midlesion and oriented to capture the collagen meniscal implant once introduced. The implant is introduced via the ipsilateral working portal using a specially designed delivery mechanism and subsequently guided by the initial suture, which serves to lasso and temporarily secure the meniscal implant. Vertical mattress sutures are placed to secure the implant to the rim of the existing meniscus. The implant is secured to the anterior and posterior horn of the existing meniscus via horizontal mattress sutures (Figs. 36-3 and 36-4). Use of 2-0 nonabsorbable sutures is recommended. After the implant is properly secured to the existing meniscal tissue, the initial temporary suture is removed and the newly placed meniscal implant is checked for fixation integrity (Fig. 36-5).30
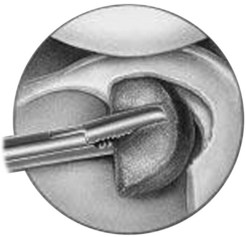
Figure 36-2 Illustration depicting insertion of the collagen meniscal implant.
(From Stone KR, Steadman JR, Rodkey WG, Li ST: Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am 79:1770–1777, 1997.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree