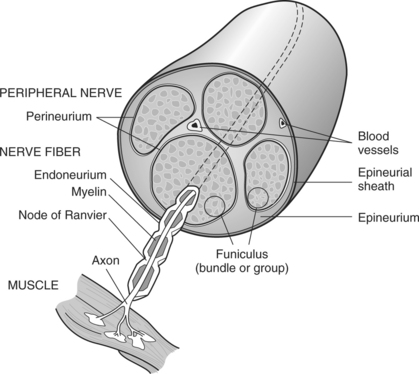
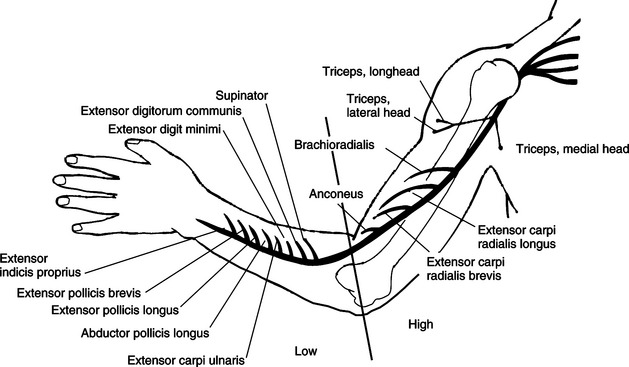
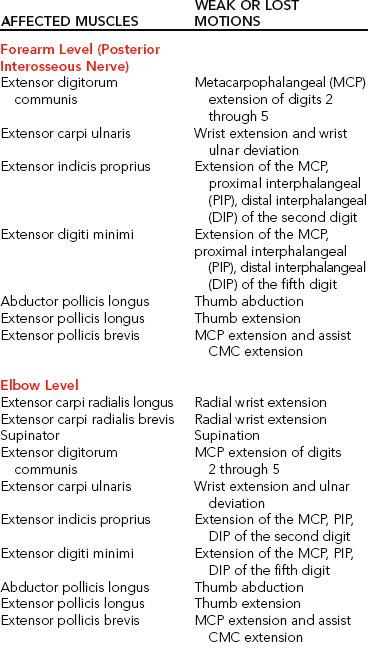
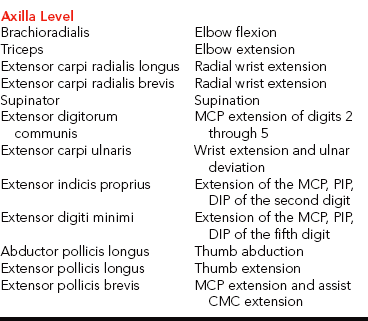
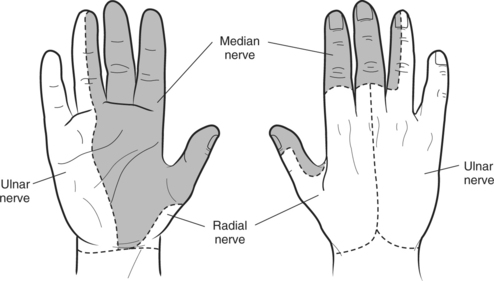
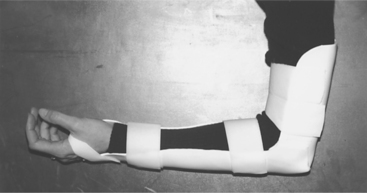
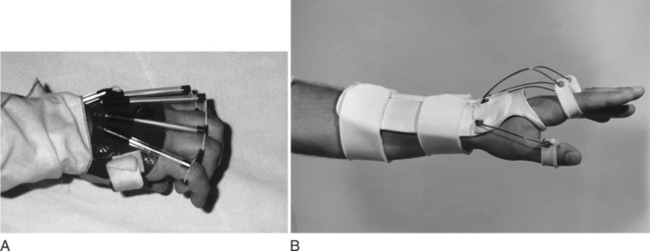
CHAPTER 13 1 Identify the components of a peripheral nerve. 2 Describe a peripheral nerve’s response to injury and repair. 3 List the operative procedures used for nerve repair. 4 List the three purposes for splinting nerve palsies. 5 Describe the nerve injury classification. 6 Identify the locations for low and high peripheral nerve lesions. 7 Explain causes of radial, ulnar, and median nerve lesions. 8 Review the sensory and motor distributions of the radial, median, and ulnar nerves. 9 Explain the functional effects of radial, ulnar, and median nerve lesions. 10 Identify the splinting approaches and rationale for radial, ulnar, and median nerve injuries. 11 Use clinical judgment to evaluate a problematic splint for a nerve lesion. 12 Use clinical judgment to evaluate a fabricated hand-based ulnar nerve splint. 13 Apply documentation skills to a case study. 14 Understand the importance of evidence-based practice with provision of splints for nerve conditions. Splint interventions for nerve lesions require that therapists have a thorough knowledge of static (immobilization) and dynamic (mobilization) splinting principles and sound critical-thinking skills. Comprehension of kinesiology, physiology, and anatomy is paramount to understanding the motor, sensory, and vasomotor implications of a nerve injury. Competence in manual muscle-testing skills is also necessary to evaluate the muscles as nerves recover from injuries [Colditz 2002]. This chapter includes information on peripheral nerve anatomy; nerve injury classifications; nerve repair; and types, effects, and treatments for radial, ulnar, and median nerve injuries. As shown inFigure 13-1, a peripheral nerve consists of the epineurium, perineurium, endoneurium, fascicles, axons, and blood vessels [Jebson and Gaul 1998]. The epineurium is made of loose collagenous connective tissue. There are external and internal types of epineurium. The external epineurium contains blood vessels. The internal epineurium protects the fascicles from pressure and allows gliding of fascicles. The amount of epineurium varies among persons, nerve types, and along each individual nerve. The perineurium surrounds fascicles, and the endoneurium surrounds the axons. A fascicle consists of a group of axons that are surrounded by endoneurium and are covered by a sheath of perineurium. An individual fascicle contains a mix of myelinated and unmyelinated fibers. The myelin sheath encapsulates the axon. Myelin is a lipoprotein, which allows for conduction of fast impulses. Each nerve contains a varied number and size of fascicles. Nerves are at risk for injury when laceration, avulsion, stretch, crush, compression, or contusion occurs [Callahan 1984]. In addition, peripheral nerves can be attacked by viruses, bacteria, or the body’s immune system [Greene and Roberts 2005]. Often bone, tendon, ligament, vessel, and soft-tissue injuries accompany nerve injuries. Nerve injuries are categorized by the extent of damage to the axon and sheath [Skirven 1992]. Nerve compression lesions often contribute to peripheral neuropathies. When a specific portion of a peripheral nerve is compressed, the peripheral axons within the nerve sustain the greatest injury. Initial changes occur in the blood/nerve barrier followed by subperineural edema. This results in a thickening of the internal and external perineurium [Novak and Mackinnon 1998]. As the compression worsens, the motor, proprioceptive, light touch, and vibratory sensory axons become more vulnerable [Spinner 1990]. All the fibers may be paralyzed after enduring severe and prolonged compression. Seddon [1943] originally described three levels of nerve injury: (1) neurapraxia, (2) axonotmesis, and (3) neurotmesis (Figure 13-2). Later in 1968, Sunderland extended the classification to five levels, which are termed as first-through fifth-degree injuries. Figure 13-2 The three classifications of nerve injuries are (1) neurapraxia, (2) axonotmesis, and (3) neurotmesis. A first-degree injury involves the demylination of the nerve, which temporarily blocks conduction [Boscheinen-Morrin et al. 1987, Novak and Mackinnon, 2005]. The prognosis for persons with neurapraxia is extremely good; recovery is usually spontaneous within three months [Spinner 1990]. When a second-degree injury occurs, the axon is severed and the sheath remains intact. Wallerian degeneration occurs when a nerve is completely severed or the axon and myelin sheath are damaged, and the endoneurial tube remains intact. The segment of axon and the motor and sensory end receptors distal to the lesion suffer ischemia and begin to degenerate 3 to 5 days after the injury [Jebson and Gaul 1998]. The intact endoneurial tube allows for potential regrowth for the proximal part of the nerve to reattach to the distal portion of the nerve. With the ideal scenario the rate of regeneration is approximately 1 inch per month. Complete recovery usually occurs if regeneration happens in a timely manner before muscle degeneration [Novak and Mackinnon 2005]. A third-degree injury is a more severe form of a second-degree injury with the addition of the “continuity of the endoneurial tube destroyed from a disorganization of the internal structures of the nerve bundles” [Sunderland 1968, p. 132]. Recovery is more complicated with possible delayed or incomplete axonal growth [Sunderland 1968]. Because fibers are often mismatched, clients benefit from motor and sensory reeducation [Novak and Mackinnon 2005]. At this level of injury “the involved segment is ultimately converted into a tangled strand of connective tissue, Schwann cells, and regenerating axons which can be enlarged to form a neuroma” [Sunderland 1968, p. 135]. The effects are more severe than a third-degree injury with increased neuronal degeneration, misdirected axons, and less axon survival [Sunderland 1968]. Surgical intervention is necessary to remove the neuroma (a tumor of nerve fibers and cells). A fifth-degree injury results in partial or complete severance of the axon and the sheath with loss of motor, sensory, and sympathetic function [Sunderland 1968]. Without the directional guidance from an intact endoneurial tube, misdirected axon growth may lead to a complicated recovery. Microsurgery is required to reestablish axon direction. Occasionally, grafting is necessary if the gap is too large for approximation of the two nerve ends [Spinner 1990]. Peripheral nerve lesions often occur to the median, radial, and ulnar nerves. The location of the lesion determines the impairment of sudomotor, vasomotor, muscular, sensory, and functional involvement [Boscheinen-Morrin et al. 1987]. Sometimes nerves can be compressed at more than one site, which is called double crush injury [Upton and McComas 1973, Rehak 2001]. Therefore, it is important to be aware of key diagnostic procedures to determine the extent of compression. There are four procedures used to surgically repair nerves: (1) decompression, (2) repair, (3) neurolysis, and (4) grafting [Saidoff and McDonough 1997]. Nerve decompression is the most common operation performed on nerves. An example of surgical decompression is the transection of the transverse carpal ligament to decompress the median nerve (this is also known as a carpal tunnel release). Surgical nerve repairs involve microsurgical sutures to repair the epineurium. Surgical nerve repairs are classified as primary, delayed primary, or secondary [Jebson and Gaul 1998]. A primary repair occurs within hours of the injury. A delayed primary repair occurs within 5 to 7 days after the injury. Any surgical repair performed beyond seven days is a secondary repair. The three purposes for splinting an extremity that has a nerve injury are protection, prevention, and assistance with function [Arsham 1984]. If a nerve has undergone surgical repair, the physician may initially order application of a cast or splint to place the hand, wrist, or elbow in a protective position, thus reducing the amount of tension on the repaired nerve. Avoiding tension on a repaired nerve is extremely important because results of nerve repairs are directly related to the amount of tension across the repair site [Skirven and Callahan 2002]. Prevention of contractures is important because nerve lesions result in various degrees of muscle denervation. For example, a short opponens splint prevents a contracture of the thumb web space after a median nerve injury [Fess et al. 2005]. Sometimes a client does not seek immediate medical attention after the occurrence of a nerve injury, and a resulting contracture develops and requires splint intervention. For example, if a person presents with a clawhand deformity as a result of an ulnar nerve injury the therapist may choose to fabricate a mobilizing ulnar gutter splint to remodel the soft tissues to increase passive extension of the ring and little fingers’ proximal interphalangeal (PIP) joints [Callahan 1984]. Once metacarpophalangeal (MCP) and PIP stiffness have occurred treatment should focus on regaining maximum passive range of motion (PROM). After normal PROM is reestablished, splinting interventions for the muscle imbalance become an option [Fess 1986]. Cumulative trauma disorder (CTD) is not a medical diagnosis but an etiologic label for a range of disorders [Melhorn 1998]. The cause of CTD is not solely work activities. Social activities, activities of daily living (ADL), and leisure pursuits may also enhance the development and exacerbation of CTD [Melhorn 1998]. The first step in controlling the CTD is to understand the compressive neuropathies of the upper extremity [Vender et al. 1998].Table 13-1 outlines the nature and treatment of compressive neuropathies that can occur at the wrist, elbow, and forearm. The compressive neuropathies are discussed in more detail later in this chapter. The location of a nerve lesion determines the sensory and motor result. Lesions are referred to as low or high. Low lesions occur distal to the elbow, and high lesions occur proximal to the elbow [Barr and Swan 1988]. High lesions affect more muscles and may affect a larger sensory distribution than low lesions. Therefore, knowledge of relevant anatomy is important. When a nerve lesion occurs, “there is no opposing balancing force to the intact active muscle group” [Colditz 2002, p. 622]. If a nerve lesion remains unsplinted, the intact musculature overpowers the denervated muscles. Intact musculature takes over and produces movement normally generated by the dennervated muscles [Clarkson and Gilewich 1989]. The person learns to adapt to the imbalance [Posner 2000, Colditz 2002]. An example of a substitution or trick movement is the pinch that develops after a low-level median nerve injury. With the help of the adductor pollicis, the flexor pollicis longus pinches objects against the radial side of the index finger. A therapist may mistakenly think that motor return has occurred for the abductor pollicis brevis, flexor pollicis brevis, opponens pollicis, and first and second lumbricals. However, the pinch movement observed is actually a substitution. Many factors affect the prognosis of recovery from a nerve injury. These factors include the extent of the injury, the cleanliness of the wound, the method of repair, and the client’s age [Skirven 1992, Skirven and Callahan 2002]. Other factors that alter nerve repair include the amount of tension on the repair, the person’s general health, and whether the person smokes. Correct alignment of axons and avoidance of tension on the damaged nerve improve the prognosis. A clean wound has a better prognosis than a dirty wound [Boscheinen-Morrin et al. 1987]. Sharply severed nerves recover better than frayed nerve damage resulting from a crush or gunshot wound [Frykman 1993]. Nerve microsurgery “timed appropriately according to the nature and extent of the injury is essential for a favorable outcome” [Skirven 1992, p. 324]. Age is also a factor in the speed of recovery. A child’s potential for regeneration is greater than an adult’s [Skirven 1992]. Full sensory and motor return occurs often in a child but rarely in an adult. Radial nerve palsies are very common and typically occur from midhumeral fractures or compressions [Arsham 1984, Colditz 1987]. Other causes of superficial radial nerve palsies at the wrist include pressure, edema, and trauma on the nerve from crush injuries; de Quervain’s tendonitis; handcuffs; and a tight or heavy wristwatch [Eaton and Lister 1992]. The location of the radial nerve injury determines which muscles are affected (Figure 13-3). Three lesions are possible when the radial nerve is injured [Colditz 2002]. The first type of lesion involves a high injury at the level of the humerus that results in wrist drop and lack of finger MCP extension (Figure 13-4). With this type of lesion, the triceps are rarely affected unless the injury is extremely high. The second type of lesion involves the posterior interosseous nerve. After spiraling around the humerus and crossing the elbow, the radial nerve divides into a motor and a sensory branch [Eaton and Lister 1992]. The motor branch is the posterior interosseous nerve, and the sensory branch is the superficial branch of the radial nerve. Compression usually causes this palsy, but lacerations or stab wounds can also be sources of lesions to the posterior interosseous nerve. Radial tunnel syndrome and posterior interosseous nerve compression are two distinct types of compression syndromes that can occur in the same tunnel and with the same nerve. As Gelberman et al. [1993, p. 1870] state, “It is difficult for the conscientious diagnostician to accept the reality that the same nerve compressed in the same anatomical site can result in two entirely different symptom complexes.” Compression of the radial nerve just distal to the elbow between the radial head and the supinator muscle is typically called radial tunnel syndrome [Izzi et al. 2001, Skirvern and Callahan 2002] and is linked to repetitive forearm rotation [Cohen and Garfin 1997]. With radial tunnel syndrome, complaints of pain are usually in the radial nerve distribution of the distal forearm [Hornbach and Culp 2002] and will involve sensory problems without muscle weakness [Eaton and Lister 1992, Gelberman et al. 1993]. Posterior interosseous nerve compression results in rapid motor loss [Gelberman et al. 1993], with no sensory loss [Eaton and Lister 1992, Gelberman et al. 1993, Kleinert and Mehta 1996]. It is characterized by aching on the lateral side of the elbow, difficulty with MCP finger and thumb extension, and difficulty with thumb abduction. Wrist extension is intact, but the wrist tends to radially deviate due to muscle imbalance [Kleinert and Mehta 1996]. The third type of lesion is damage to the sensory branch of the radial nerve. This type of lesion does not result in a functional loss. However, compression symptoms include numbness, tingling, burn, and pain over the dorsoradial surface of the hand [Skirven and Osterman 2002]. Compression of this superficial branch is called Wartenberg’s syndrome [Nuber et al. 1998]. Table 13-2 outlines the muscles and motions that are affected and the lesion locations in radial nerve lesions. After crossing the elbow and dropping below the supinator, the radial nerve divides and forms the posterior interosseous nerve [Colditz 2002]. Lesions and compressions of the posterior interosseous nerve at the forearm level can affect the following muscles: In addition to the motions lost at the forearm level, an injury at the elbow level involves a loss of radial wrist extension, MCP joint extension, thumb extension, thumb radial abduction, and weakened forearm supination. The functional results of an axilla-level lesion are a loss of wrist stabilization in an extended position, loss of finger and thumb extension, and loss of thumb abduction. A client with a high radial nerve lesion has poor grip and coordination because of the lack of wrist extensor opposition to the flexors [Fess 1986, Bosheinen-Morrin et al. 1987]. The resulting deformity is called wrist drop. Significant loss of sensation is not present with radial nerve injuries. The superficial sensory branch of the radial nerve supplies sensation to the dorsum of the index and middle fingers and half of the ring finger to the PIP joint level.Figure 13-5 shows a representation of hand sensory distribution from the radial nerve. Laceration or contusion to the sensory branch of the radial nerve can be annoying. This often occurs in conjunction with de Quervain’s release. Sensory compromise over the dorsum of the thumb may result in hypersensitivity. Sometimes a splint or padded device can protect the area while a desensitization program is implemented [personal communication, K. Schultz-Johnson, October 1999]. For this condition, the elbow is positioned in approximately 90 degrees flexion, forearm in full supination, and the wrist in slight wrist extension (20 to 30 degrees) [Gelberman et al. 1993, Alba 2002]. Positioning the forearm in supination decompresses pressure on the radial nerve. The splint (Figure 13-6) is worn all the time, with removal for hygiene [Alba 2002]. Kleinert and Mehta [1996] suggest the splinting approach of fabricating a thermoplastic thumb immobilization splint (see Chapter 8). A couple of splinting options are suggested for posterior interosseous nerve syndrome. One option is to fabricate a long-arm elbow and wrist splint with the elbow in flexion, forearm in neutral or slightly supinated, and the wrist in 20 to 30 degrees of extension. Another option is to fabricate a tenodesis splint because it encourages wrist and finger function [Eaton and Lister 1992]. The tenodesis splint is discussed later in this chapter. For Wartenberg’s neuropathy, a wrist immobilization splint is fabricated with the wrist in 20 to 30 degrees of extension. If pain occurs with thumb motion, the thumb is also incorporated into the splint. Refer to Chapter 8 on how to fabricate a thumb splint. The therapist can use a wrist immobilization splint to place the wrist in a functional position of 30 degrees of extension [Cannon et al. 1985]. A client can usually extend the fingers to release an object by using the intrinsic hand muscles [Boscheinen-Morrin et al. 1987]. The therapist keeps in mind the advantages, disadvantages, and patterns of volar and dorsal wrist splints (see Chapter 7). A wrist immobilizer splint may be appropriate to wear on occasions when the client desires a more inconspicuous design than a mobilization splint. A wrist immobilization splint may also be more appropriate for nighttime wear than a mobilization splint. Mobilization splinting for a radial nerve injury promotes functional hand use [Borucki and Schmidt 1992]. The therapist fabricates a dorsal wrist immobilizer splint as the base for a mobilization extension splint (using elastic for the source of tension) [Arsham 1984]. The dynamic component for this splint positions the MCPs in extension. Several low-profile options exist that can be made with purchased outrigger parts (Figure 13-7). However, Colditz [2002, p. 633] remarks that “one should be cautioned against designs for dynamic wrist and finger extension, because the powerful unopposed flexors often overcome the force of the dynamic splint during finger flexion.”
Splinting for Nerve Injuries
Peripheral Nerve Anatomy
Nerve Injury Classification
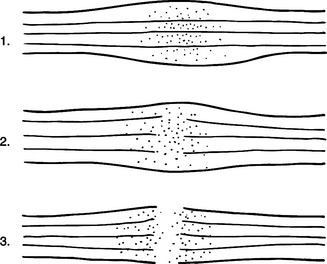
First-degree Injury
Second-degree Injury
Third-degree Injury
Fourth-degree Injury
Fifth-degree Injury
Nerve Repair
Operative Procedures for Nerve Repair
Purposes for Splinting Nerve Injuries
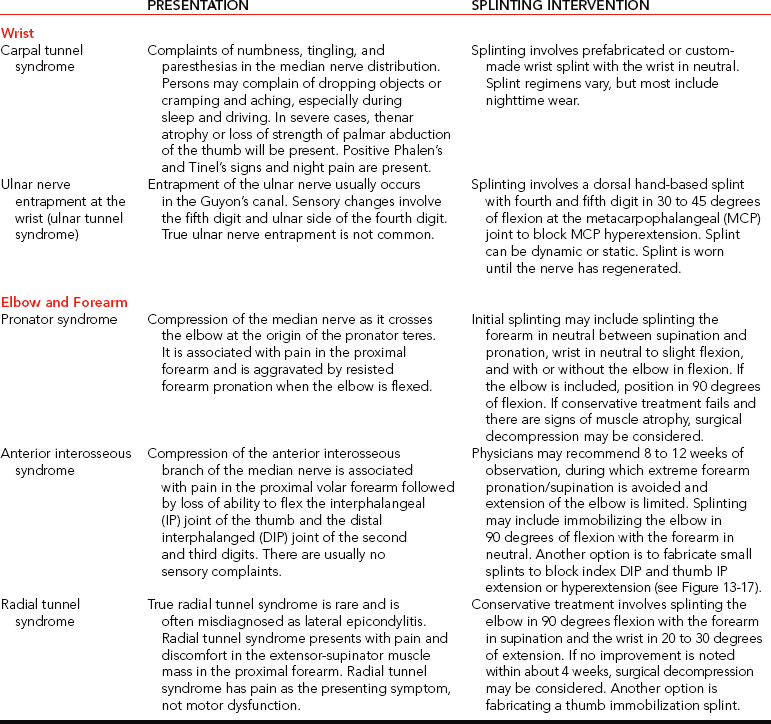
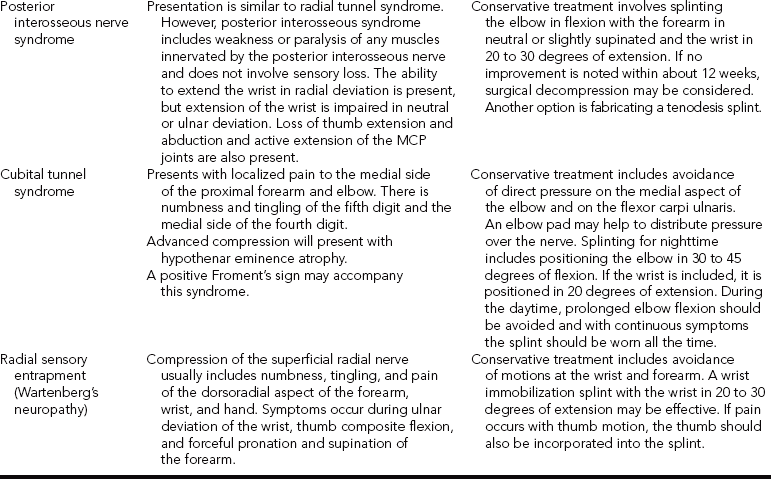
Upper Extremity Compression Neuropathies
Locations of Nerve Lesions
Substitutions
Prognosis
Radial Nerve Injuries
Functional Involvement from Radial Nerve Lesions
Radial Nerve Injury Splint Intervention
Splinting for Radial Tunnel Syndrome
Splinting for Posterior Interosseous Nerve Syndrome
Splinting for Wartenberg’s Neuropathy
Wrist Immobilization Splint
Mobilization Extension Splints
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Splinting for Nerve Injuries