Chapter 16 Spinal Cord Mechanisms of Referred Pain and Related Neuroplasticity
Convergence-projection, supersegmental, convergence, neuroplasticity, central sensitization, facilitated state, long-term potentiation, phasic inhibitory control, tonic inhibitory control
After reading this chapter you should be able to answer the following questions:
| Question 1 | What is the contribution of central neuronal plasticity and referred pain? |
| Question 2 | What mechanism may account for ongoing back pain independent of proprioceptive afferent input from the periphery? |
| Question 3 | By what mechanism can spinal manipulation inhibit low back pain? |
I. Referred pain and hyperalgesia from deep tissue damage
II. Clinical phenomenology of referred low back pain
III. Neurobiologic explanations of referred pain
IV. Neurophysiologic evidence for central convergence
V. The contribution of central neuroplasticity to referred back pain and hyperalgesia
VI. Sympathetic nervous system and low back pain
VII. Neurophysiology of paraspinal antinociceptive mechanisms
Most clinically significant pains are triggered by damage to deep somatic and visceral tissues. For many years clinicians have observed that when tissues lying deep to the skin are injured, inflamed, or diseased, the patient reports that not only is the local area of damage painful but surrounding and sometimes distant uninjured areas are also painful; this phenomenon is called pain referral.1–6 Referred pains are often felt in, or referred to, structures innervated by the same spinal cord segment as that innervating the damaged structure (isosegmental convergence); however, the painful region often expands or spreads (radiation of pain) to tissue areas served by other adjacent spinal segments.1–4,7 This dynamic change in the perceived area of pain is usually triggered by continued or recurrent injury of the original site of deep tissue damage.1–4
The patient with deep tissue referred pain also has difficulty locating the source(s) of his or her pain, in contrast to a skin injury, the pain of which is seldom if ever mislocated by the patient.1,3,4 As a general rule, the deeper and the more proximal the damaged structure, the greater the degree of pain mislocation by the patient.2–4 Referred pain also arises rapidly and becomes maximal only a few minutes after deep somatic or visceral tissue injury and may last from hours to days in normal individuals1,2 or for much longer periods (weeks, months, years) in symptomatic patients.1–4
Besides being difficult to localize, pains of deep somatic or visceral origin have a dull aching quality and are often associated with tissue tenderness that results in exaggerated painful sensation on palpatory stimulation (called referred tenderness or hyperalgesia).1–3 This referred tenderness, felt locally in the area of injury, can be provoked also in the area of referral where the tissues are normal. Interestingly, local anesthetic injections into the referral area blocks the referred hyperalgesia but not the local tenderness, whereas injections into the area of deep injury block both the local and the referred tenderness.2–4 These findings suggest that painful input from the damaged deep tissues is crucial for the production and elaboration of both the local and the referred hyperalgesia.
Because deep referred pains are poorly localized by the patient, it is difficult for the attending physician to diagnose the exact source and cause of the patient’s problem. This is especially so when there is deep tissue damage of axial structures like the back (or head), but it is seldom a problem in the case of an extremity injury like a sprained ankle.1,2 Because of the importance of the symptom of referred back pain and its alleviation in clinical chiropractic practice, and because of the recent appearance of uniquely new findings on the lumbar spine pain-signaling system,8–13 this chapter focuses on the neurophysiologic correlates of lumbar spinal pain and referral.
Clinical Phenomenology of Pain Originating from the Lumbar Spine
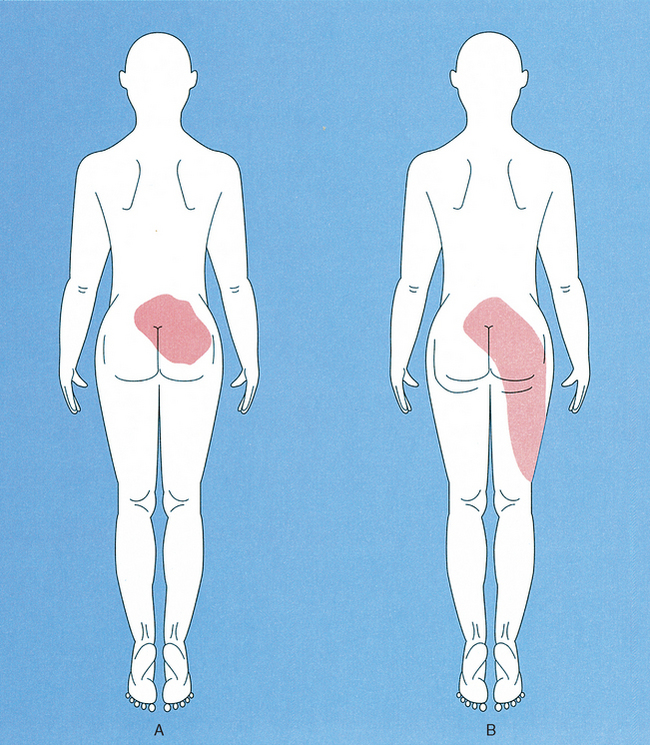
Commonly, chiropractic physicians are asked to help patients whose primary complaint is back pain and, more specifically, low back pain (Figure 16-1).14 The crucial initial clinical question for determining diagnosis and subsequent treatment is “Where is the pain coming from?” Traditionally, the back pain is assumed in most instances to arise from previous or ongoing injury or disease of lumbar “motion-segment structures” such as facet (zygapophyseal) joints, muscles, ligaments, intervertebral discs, bone or periosteum, meninges (dura), and associated vascular elements.1–23 (See Chapter 2.) Indeed, numerous clinical investigations have shown that all of these spinal tissues, when appropriately stimulated, can give rise to generalized low back and referred leg pain (called referred or nonradicular pain) in normal or symptomatic individuals, and local anesthetic blockage of these same structures or their neural innervations can eliminate such pain.* Typically, the pain arising from these structures is poorly localized to the low back and hip region bilaterally and is reported as having an aching quality felt deep to the skin surface (Figure 16-1, A). In addition, associated abnormal skin sensations are sometimes reported, including hyperalgesia to mechanical and thermal stimuli.2,25,26
In addition to these features, the low back pain patient frequently reports that the pain spreads (radiates) from the back into the hip and leg as the condition continues to worsen (Figure 16-1, B).† The radiating leg pain is often called sciatica2,19,22,23 and should be clinically distinguished from the superficially localized “electric shock-like” leg and foot pain arising from segmental nerve root compression (called true radicular pain).19,23 Radicular and nonradicular (referred) leg pain are currently believed to arise from quite different pathophysiologic processes.1,2,19,23
Explanatory Hypotheses
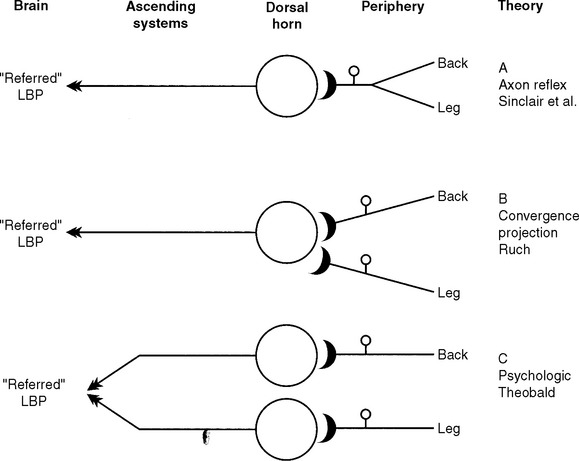
Neurobiologic explanations of referred pain have historically rested on some anatomic feature of the peripheral innervation, an organizational feature of the spinal cord, or some process occurring in the brain (Figure 16-2). The clearest formulation of a peripheral explanatory hypothesis of pain referral was that of Sinclair et al.6 published some decades ago (Figure 16-2, A). They speculated that
…the factor in the production of referred pain is the existence of branching among the sensory pathways conveying the sensation of pain. This branching is of such a type that one limb of a branched axon passes to the site of origin of the disturbance and the other passes to the sites to which the pain is referred (axon reflex). This mechanism works in two ways: first by leading to a misinterpretation by the central nervous system of the true origin of the pain impulses and secondly by the liberation of metabolites at the terminals in the region where the referred pain is experienced, thus giving rise to secondary pain impulses actually having origin at the periphery.6
Evidence suggests that this explanation is inadequate for pain referral in general and referred lumbar pain in particular for at least three reasons. First, the patient’s report of the location of the back pain usually involves widespread areas of the back, hip, and legs that are served by completely separate and non-overlapping peripheral neural innervations.16,18,19,23 Second, the peripheral branching of somatosensory pain afferents with one branch in back tissues and the other branch innervating the hip or leg is yet to be confirmed experimentally.1,27 Third, and most important, this type of “static” anatomic explanation cannot account for the consistent clinical observation that these pains are “dynamic,” that the painful area grows in size and spreads into uninjured tissue regions (for example, hip and leg) served by other spinal segments as the condition worsens or is exacerbated by reinjury (Figure 16-1, B).* All of these considerations argue against the peripheral axon reflex mechanism as an explanation for referred deep tissue pains in general and low back pain in particular.
If peripheral innervation is unable to explain the clinical symptomology, then where do we go to find a more satisfactory explanation? An obvious place to look is within the central nervous system itself. Because of the uniformity of the pain pattern produced by provocative stimulation of lumbar paraspinal structures, Livingston4 and later Kellgren19 suggested that there must be an underlying population of pain-signaling neurons that receive convergent input from all of the clinically relevant deep spinal sources. Moreover, they argued that when these neurons are driven to activity by painful (nociceptive) injury messages coming from any injured spinal tissue, the brain interprets the projected impulse activity of these cells to mean that pain is originating from all of the tissue areas having potential connections with these neurons, and so the pain is “referred” to a much larger region than the actual area of injury. This idea, called the convergence-projection theory of pain referral (see Figure 16-2, B), had originally been suggested by Ruch5 to explain pain referral of visceral disease to somatic tissues of the body. Even though ample neurophysiologic evidence supporting somatovisceral convergence-projection at the spinal cord level has appeared in recent years,1,7,28 a search for somatosomatic convergence in pain-signaling neurons serving the vertebral column has never been attempted until recently. A consideration of these issues prompted experiments in animals (rats and cats) to see if spinal cord neurons could be found that show convergence of nociceptive input from the low back, hip, and leg region, as originally suggested by Kellgren19 and others.2,4,18,22 This evidence is discussed in detail in the next sections of this chapter.
Finally, Theobald29 many years ago postulated that deep tissue pain and referral might result from the processing of neuronal impulse traffic from the damaged and referral territories only at psychological (that is, suprasegmental) levels (Figure 16-2, C). This possibility has received only limited experimental analysis, yet there is no question now that single nerve cells in the somatosensory thalamus and neocortex show the requisite evidence of interaction of peripheral inputs. However, the available evidence relates only to somatic tissues of the extremities.30 Because nothing is known about how suprasegmental regions of the somatosensory pathway process pain input from the vertebral column, our appraisal of the neurobiologic evidence related to referred low back pain is restricted to that obtained at the spinal cord level.
Neurophysiologic Evidence for Central Convergence-Projection
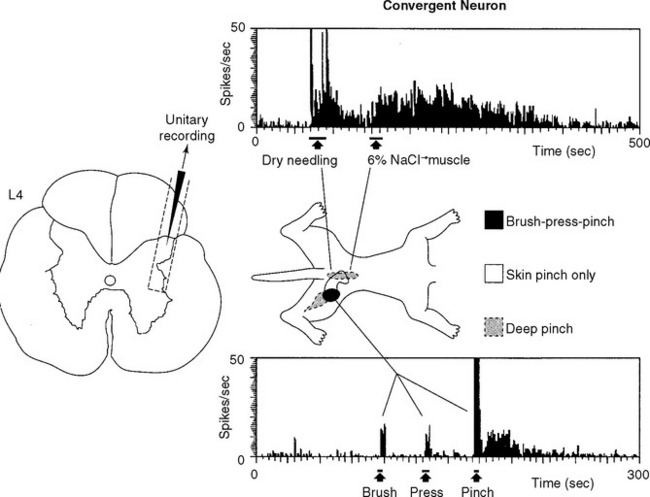
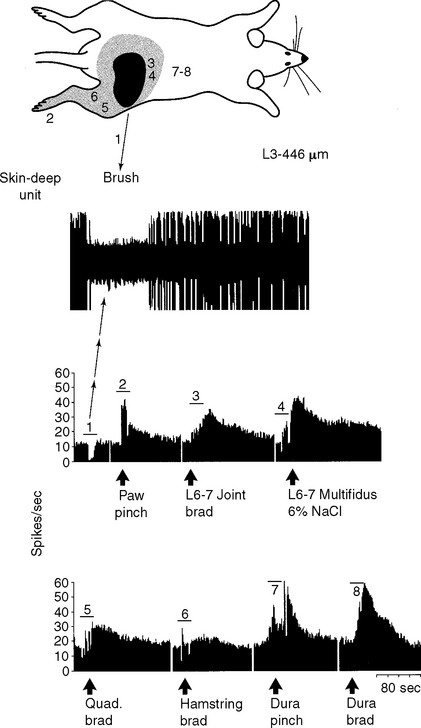
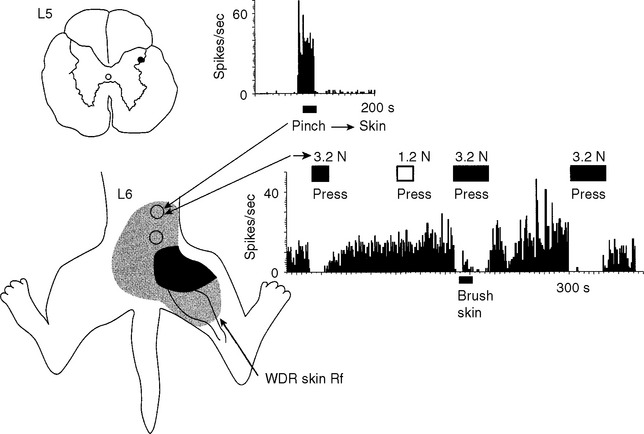
In our initial experiments, we have recorded from individual somatosensory neurons within the spinal cord that respond to tissue damage in the low back region, neurons likely to mediate low back pain.8,10,11 We found that most of these “low back” pain-signaling neurons, located exclusively in the lateral dorsal horn of the lumbar cord (Figure 16-3), were responsive to noxious, damaging stimuli applied to many different tissues of the low back, hip, and proximal leg; that is, within the “receptive field” of the cell. Recall that a neuron’s receptive field is that area of tissue within which peripheral stimulation causes the neuron to generate an action potential, that is, the neuron receives information from that tissue.1,7 Because of the remarkable input convergence to these cells from multiple back and hind limb tissues, we now refer to these neurons as “hyperconvergent” low back pain neurons (hereafter, “low back neurons”).
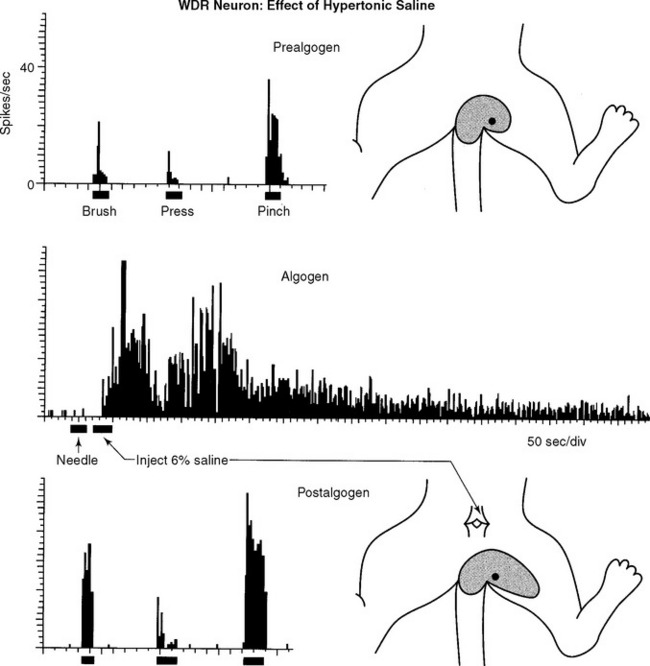
Individual low back neurons were found to be either responsive to noxious and nonnoxious stimulation (multireceptive or wide-dynamic range [WDR] neurons, 77%) or noxious stimulation only (high-threshold or nociceptive-specific [NS] neurons, 23%) of paraspinal muscles, ligaments, discs, periosteum, facet joints, dura, and skin, as well as tissues of the hip and leg (Figures 16-3 and 16-4). Evidence strongly suggests that WDR neurons are responsible for the generation of subjective pain in humans and NS units appear not to contribute to this sensation.7 In addition, all of the WDR and NS neurons that we examined were found to be more powerfully activated by nociceptive input from deep tissues than from skin, which we believe may explain why back pain is felt to originate from deep rather than superficial tissues. We have also established that some of the recorded neurons (for example, units shown in Figures 16-3, 16-4, 16-8, and 16-10) project axons into well-recognized ascending pain pathways (for example, the spinothalamic, spinoreticular, and spinocervical tracts).8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree