6 Key Points 1. The outcome of SCI greatly correlates with the extent of neurological injury and initial functional impairment. 2. The simplest and most useful method of classification of acute SCI is into complete and incomplete injuries. The ASIA classification is the most widely used international system to document sensory and motor impairment following SCI. 3. The ZPP applies to complete SCI or ASIA A and refers to all segments below the neurological level of injury with some preservation of motor or sensory function. Over time, some patients may spontaneously recover functional motor strength within the ZPP. Spinal cord injuries (SCIs) constitute a major source of social and economic burden to our society. Every year, more than 12,000 Americans suffer an SCI as a direct result of trauma.1 Over the past decades, significant progress has been made in the evaluation and management of patients with SCI; furthermore, most SCI clinicians and researchers maintain an investigative mindset that continuously fuels further improvement of SCI assessment and treatment. The initial neurological evaluation of SCI patients is critical because it dictates the acute management and determines the long-term prognosis. It is crucial to identify the level as well as the completeness of an SCI. Based on the American Spinal Injury Association/International Medical Society of Paraplegia (ASIA/IMSOP) standards, the level of injury is defined as the most caudal level of normal motor and sensory function.2 The simplest and most useful method of classification of acute SCI is into complete and incomplete injuries. Because the outcome for SCI patients greatly correlates with the extent of neurological injury and initial functional impairment, it is important to have a reliable and reproducible method of classification of SCI. Several grading systems have been produced over the years. Clinicians have used various scales to grade neurological deficits following SCI. In 1969, Frankel and colleagues3 published the first classification system for acute SCI based on a study conducted in Stoke Mandeville Hospital over a period of 10 years. They retrospectively evaluated 682 SCI patients and designed a five-grade system based on the neurological function of these patients. Grade A designated patients with complete loss of motor and sensory function below the level of injury, including loss of sacral function. Grade B was for patients with complete loss of motor function but sensory preservation including sacral sparing. Grades C and D described patients who had retained some motor function; however, this motor function was judged useless in grade C as opposed to useful in grade D. Patients assigned to grade E had full neurological function. Following its publication, the Frankel scale was widely used; it was easily applicable and was uniquely based on gross motor and sensory evaluation of patients. However, it had significant limitations; for instance, it did not clearly segregate patients in groups C and D. The Frankel scale was also not sensitive enough to reflect significant motor function improvement of a patient into a better Frankel grade.4,5 Several modifications of the Frankel scale were published in the following years, but they all lacked sensitivity.6–9 Since the establishment of the Frankel classification system, several others have followed. Bracken et al.10 designed a grading system in 1978, based on a prospective study of 133 SCI patients at the Yale University School of Medicine. They segregated motor and sensory neurological deficits into a five-scale and a seven-scale system, respectively. The motor scale included the following categories: (1) antigravity movement in all myotomes, (2) and (3) trace contraction in some muscles of a myotome, (4) absence of contraction at or below the iliopsoas, (5) absent contraction in the first palmar interosseus or higher. The sensory scale was based on pinprick evaluation and listed seven categories: (1) normal, (2) some segments decreased, (3) some segments absent, (4) paraparesis, (5) quadriparesis, (6) paraplegia, and (7) quadriplegia. This scale demonstrated a strong correlation between changes in motor and sensory scores and patients’ outcome at discharge. However, it had a significant flaw in that cross-classification of the motor and sensory scales showed a discrepancy. This system fell out of favor because it was difficult to memorize and perform at the bedside. It also failed to integrate motor and sensory exams and did not account for sacral function. Lucas and Ducker11 at the Maryland Institute for Emergency Medical Services also published a motor classification system in the late 1970s. They conducted a review of 436 SCI patients with a single-level vertebral trauma and proposed a classification based on the motor index initial (MIi). The MIi was defined as the sum of motor function for each muscle group tested, divided by the number of motor groups tested (up to 14). Motor function was graded 0 to 5, with 5 normal, 4 functional, 3 fair, 2 poor, 1 trace, and 0 absent. In their series, MIi was directly related to recovery rate. This classification system was never widely used, but it introduced a standardized method of motor examination that inspired subsequent grading scales.8 Several other classification systems were introduced in the early 1980s. Klose and colleagues12 published the University of Miami Neuro-spinal Index (UMNI), which combined both sensory and motor scales. Motor function was recorded by testing 44 muscle groups. A score of 0 was given for no function, 1 for flicker of contraction, 2 for movement without gravity, 3 for movement against gravity, 4 for movement overcome by resistance, and 5 for normal power, for a total of 0 to 220 points. The sensory function was tested by evaluating pinprick and vibration on the right and left sides of the body at all 30 segments of the spinal cord. A score of 0 was given for absent sensation, 1 for present but abnormal, 2 for normal, for a total of 0 to 240 points. This classification was found useful because it had good interrater reliability and could be used to closely monitor patients’ progression. However, it was cumbersome to perform and compute and did not evaluate sacral function. In 1981, Chehrazi and colleagues13 developed a classification scale also known as the Yale Scale. This scale was a composite of numerical motor and sensory scales and assessed the severity of SCI and prognosis for recovery. The motor scale was computed by averaging the strength of muscle groups below the injury level, graded 0 to 5 as described by the Royal Medical Research Council of Great Britain (Table 6.1). The sensory scale was the average of pinprick, position, and deep pain senses. Pinprick and position were graded 0 to 2 in dermatomes below the level of injury. Deep pain was assessed by compression of the Achilles tendon or toe, with 1 given for correct localization and 0 for no localization. A total of 0 to 10 points could be achieved, with 0 indicating complete absence of motor and sensory function and 10 intact motor and sensory function. This classification system had good reliability and was easily memorized and performed at the patient’s bedside. However, like most previous scales, it failed to evaluate sacral function. In 1984, ASIA held a conference in Chicago to define standards for the neurological classification of SCI patients. The neurological assessment focused on the examination of 10 muscle groups, five in the upper extremities and five in the lower extremities, graded 0 to 5 points. Sensory evaluation was only recorded as the most cephalad level of normal sensation, and the Frankel classification was used to grade the functional status of patients. In 1989, the ASIA standards were revised to provide better sensory examination,5 with the assessment of light touch and pinprick sensation. In 1991, Priebe and Waring14 evaluated the interobserver reliability of the 1984 and 1989 ASIA grading systems. They found that the 1989 ASIA standards had greater accuracy, but they still had a less than optimal coefficient of reliability (k = 0.67). In 1992, ASIA standards for the neurological classification of SCI patients were revised for a second time, in association with the IMSOP15 (Fig. 6.1 and Table 6.2). In addition to the previous assessments of motor function, sensory level, and Frankel functional impairment, the new standards incorporated the Functional Independence Measure (FIM). The FIM was developed in an effort to provide a uniform appraisal of disability for SCI patients.7,16 It evaluates patients’ functional status based on their ability to perform activities of daily living, including self-care, control of bowel and bladder function, ambulation, as well as social interaction. The advantage of using the FIM over time is that it records socioeconomically meaningful improvements in the neurological function of SCI patients. Subsequent studies of the ASIA/IMSOP scale found that it provided a good discrimination in severity of SCI and predictability of outcome.17,18 However, it was found to have weak interobserver reliability, principally for the grading of incomplete SCI.19,20 Additional updates were made to the ASIA/IMSOP standards in 1996, with inclusion of the ASIA Impairment Scale, the ASIA Motor Index Score, the ASIA Sensory Scale, and the FIM. These updates further refined the assessment and classification of acute SCI and are widely recognized and used.
Spinal Cord Injury Classification
 History of Spinal Cord Injury Classification
History of Spinal Cord Injury Classification
First Classification System: Frankel Grade
Evolution of Classification Systems
American Spinal Injury Association/International Medical Society of Paraplegia Standards
Grade | Muscle strength |
0 | Complete paralysis |
1 | Flicker |
2 | Movement with gravity eliminated |
3 | Movement against gravity but no resistance |
4 | Movement against gravity and resistance |
5 | Full strength |
Table 6.2 American Spinal Injury Association Impairment Scale
A | Complete: no motor or sensory function, including in sacral segments |
B | Incomplete: Sensory but no motor function is preserved below the level of injury, including in sacral segments |
C | Incomplete: motor function is preserved below the level of injury, and the majority of key muscles below the level of injury have a muscle grade < 3/5 |
D | Incomplete: motor function is preserved below the level of injury, and the majority of key muscles below the level of injury have a muscle grade ≥ 3/5 |
E | Normal: motor and sensory functions are normal |
Source: The American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, Reprint 2008. Chicago, IL. Reprinted with permission.
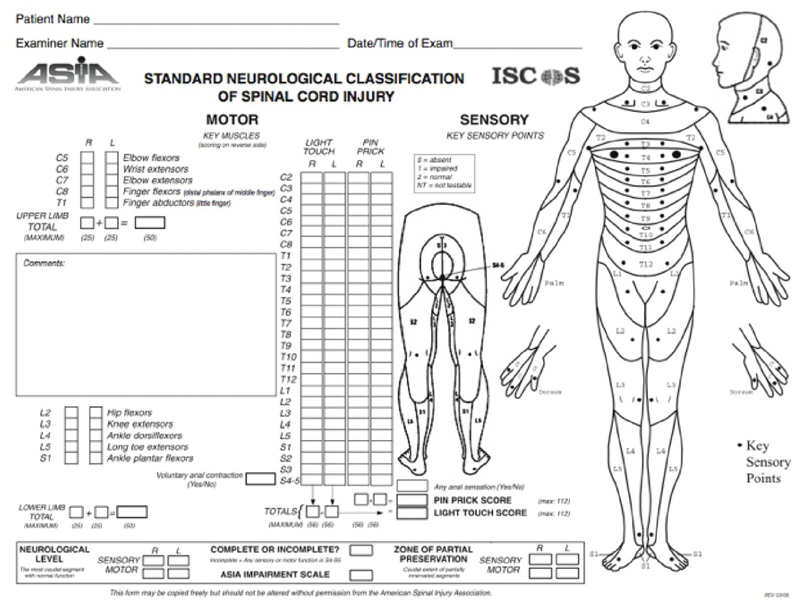
Fig. 6.1 American Spinal Injury Association Standard Neurological Classification of Spinal Cord Injury
 Functional and Anatomical Classification of Spinal Cord Injury
Functional and Anatomical Classification of Spinal Cord Injury
Level of Injury: Skeletal versus Neurological
The relationship and numbering or labeling of the osseous spinal column and the spinal cord parenchyma can be confusing. As humans develop from infancy to adulthood, the spinal column undergoes greater rostrocaudal growth than the spinal cord. This results in the distal spinal cord being stretched from the sacrum to approximately L1 osseous spinal column level. The spinal cord parenchymal level (neurologic level) then is displaced from its original osseous level. This can result in a patient having an osseous injury but a different neurologic level of injury. In the upper cervical area, spinal cord segments overlay the same number of vertebral bodies; however, as one progresses caudally to the thoracic area, spinal cord segments overlay vertebral bodies of one then two levels below. Ultimately, the T11 vertebral body corresponds to the L1 spinal cord segment, and the conus medullaris is located between L1 and L2 vertebral bodies. For example, a T11 burst fracture often results in a patient having a neurologic injury level of L1.
The skeletal level of injury refers to the level of maximal vertebral column damage on the radiograph. The neurological level of injury is the most caudal level at which motor and sensory functions are intact. Depending on the location of the injury, the skeletal level may correspond to the neurological level; however, it may also correspond to the neurological level one to two segments below. In the assessment of acute SCI, it is important to record a sensory level, which is the most caudal dermatome with 2/2 score for light touch and pinprick, as well as a motor level, which is the most caudal key muscle with a strength of 3 or above while the segment above is normal or 5/5, for the left and right side of the body independently.
Complete Spinal Cord Injury (ASIA A)
Patients suffering from a complete SCI have no preservation of motor and sensory function below the level of injury, including in the sacral segments. These patients are defined as ASIA A. Complete SCI above the C3 level can result in death from respiratory failure without immediate intervention with cardiopulmonary resuscitation (CPR). Patients with complete SCI can also develop loss of autonomic function and spinal shock. In addition, they can have bowel and bladder dysfunction with incontinence in the acute posttraumatic phase. At a later time they become hyperreflexic, and unlike incomplete SCI, very few patients with complete SCI will recover meaningful neurological function.21,22
Zone of Partial Preservation
The zone of partial preservation (ZPP) applies to complete SCI or ASIA A and refers to all segments below the neurological level of injury with some preservation of motor or sensory function. Most patients with complete SCI will have a ZPP of varying size a few segments below the neurological level. Over the period of time following the injury, some patients may spontaneously recover functional motor strength within the ZPP.
This concept becomes crucial in the evaluation of efficacy of treatment modalities for SCI patients, because a clear distinction should be made between spontaneous recovery within the ZPP and further neurological function improvement from medical interventions.23
Incomplete Spinal Cord Injury (ASIA B, C, D)
SCIs with any residual motor or sensory function more than three segments below the level of injury are considered incomplete. Signs of preserved long-tract function include sensation or voluntary movement in the lower extremities, sacral sparing with preserved perianal sensation, and voluntary rectal sphincter contraction. Patients with incomplete SCI can be classified in syndromes based on correlation of spinal cord anatomy and presenting symptoms.
Central Cord Syndrome
Central cord syndrome (CCS) is the most common type of incomplete SCI syndrome. Its occurrence is bimodal; in younger patients it may occur following severe high-energy, high-velocity spinal column trauma, as opposed to a more common incidence in the elderly after a forward fall or other minor hyperextension injuries.24,25 CCS may occur whether or not the injury results in spinal column fracture or dislocation.26,27 In elderly patients, this is often seen in patients with cervical stenosis secondary to cervical spondylosis resulting in reduced spinal canal diameter.
The pathognomonic physical finding of CCS is a disproportionate loss of motor function in the upper extremities compared with the lower extremities. More loss of distal extremity function is typically noted compared with proximal musculature strength because CCS in the cervical region usually results in severe hand paresis. Sensory deficits are variable; one of the early manifestations of CCS can be loss of pain and temperature sensation in a capelike pattern at the level of injury. This results from the damage of crossing spinothalamic fibers. Tactile sensation remains intact because posterior column fibers are preserved. In some cases, patients develop an acute or delayed hyperesthesia or allodynia in the proximal upper extremities.
There is no neuroanatomical evidence to explain the physical finding of disproportionate upper extremity weakness in CCS. Several theories have been hypothesized, one of which is that the centermost region of the spinal cord is more susceptible to injury from cord compression and edema,28 and long tract fibers are arranged somatotopically, with upper extremity fibers central to lower extremity fibers. Evaluation of patients with CCS should include cervical spine x-ray and computed tomography (CT) to evaluate for canal stenosis, spondylosis, and fractures; cervical spine magnetic resonance imaging (MRI) to look for traumatic disk herniation, ligamentous injury, and cord edema or hematoma. Several studies have shown a correlation between the length of rostrocaudal edema and neurological deficit, as well as a gradual improvement of function with decreased cord edema over time.29,30 The presence of spinal cord hematoma is associated with a worse prognosis.31
The best treatment method for CCS remains controversial. Current practice options published by the AANS and CNS include blood pressure augmentation for cord perfusion, early reduction of fracture dislocation injuries, and surgical decompression for persistent cord compression and deterioration of function.32 Almost all patients with CCS will have some degree of neurological recovery. It usually starts in the lower extremities, followed by the bladder, proximal upper extremities, and finally fine hand movement which are most limited or absent.33 Younger patients have a significantly higher recovery rate than older patients; 97% versus 41% recovery of ambulation, respectively.25
Brown-Séquard Syndrome
Brown-Séquard syndrome refers to spinal cord hemisection injury. The most common mechanism of literal cord hemisection is penetrating trauma by a missile or a knife wound. Other mechanisms of functional cord hemisection include radiation myelopathy and hemicord compression from a large herniated disc,34,35 epidural hematoma/collection, tumor, or arteriovenous malformation.
The classical clinical presentation is ipsilateral motor paralysis from the injury to the descending corticospinal tract, and dissociated sensory loss with ipsilateral loss of proprioception and vibratory sense from injury to the ascending posterior column as well as contralateral loss of pain and temperature sensation one to two levels below the level of injury from damage to the crossing spinothalamic fibers. In addition, ipsilateral compromise of lateral columns can result in autonomic dysregulation with cutaneous hyperemia and anhydrosis. Over time, injury to the ipsilateral descending corticospinal fibers can result in spastic paralysis below the level of injury as well as hyperreflexia and Babinski response, with contralateral sparing. Lesions that cause Brown-Séquard syndrome can also injure anterior horn cells and associated nerve roots; in these instances, patients can develop flaccid paralysis, hyporeflexia, and muscle atrophy as well as anesthesia and analgesia in the corresponding segments.
Of all incomplete SCIs, Brown-Séquard syndrome has the best prognosis.36 Approximately 90% of patients will recover functional motor strength and will be able to ambulate independently.37
Anterior Cord Syndrome
Anterior cord syndrome (ACS) is also known as anterior spinal artery syndrome and is thought to result from spinal cord ischemia in the territory supplied by the anterior spinal artery. ACS is the second most common type of incomplete SCI. The mechanisms of injury are thought to be anterior spinal artery occlusion versus direct anterior cord compression from a traumatic herniated disk38 or dislodged bone fragments in cervical flexion and extension injuries.
The clinical presentation is paraplegia or tetraplegia for injuries higher than C7, from damage to the descending corticospinal, rubrospinal, and vestibulospinal tracts. Sensory deficits are dissociated, with bilateral loss of pain and temperature sensations from injury to the spinothalamic fibers but preservation of proprioception and vibratory senses from intact posterior column below the level of injury.
During the evaluation of ACS, it is essential to determine whether it results from anterior spinal artery occlusion or direct anterior cord compression because the latter may require surgical decompression. Patients should be promptly evaluated with x-ray, CT, MRI, or myelography as needed. Patients with ACS have the worst prognosis for recovery of neurological function and require longer periods of rehabilitation.36
Posterior Cord Syndrome
Posterior cord syndrome (PCS) is a rare type of incomplete SCI; in its pure form it is characterized by selective injury to the posterior column. Traumatic PCS could be attributed to an expanding extradural lesion overlying the posterior cervical canal or the ligamentum flavum buckling in the setting of cervical spondylosis. However, during the initial evaluation of pure PCS, the differential diagnosis should also include tabes dorsalis and demyelinating diseases, such as multiple sclerosis.
Patients present with characteristic loss of proprioception and vibratory sense distal to the level of injury, sometimes accompanied by pain and burning paresthesias. Often, patients will develop sensory ataxia and loss of balance during a Romberg maneuver that will increase with eye closure.
Conus Medullaris Syndrome
The spinal cord terminates into a tapered end, the conus medullaris, most commonly at the L1 vertebral body or L1-2 disk inter-space level in adults.39 This section of the spinal cord contains the lumbar and sacral nerve root segments and is prone to injury because it is located at the transition point between the relatively fixed thoracic spine and the more flexible lumbar spine.40
Injuries to the conus medullaris from disk herniation or fracture usually result in a symmetrical pattern of upper and lower motor neuron dysfunction. In the acute phase, symptoms can include bilateral flaccid lower extremity paralysis, saddle anesthesia, loss of rectal tone and volition, and urinary retention. Over time, patients may develop muscular atrophy or spasticity, positive Babinski response and hyperreflexia. Overall, patients with conus medullaris syndrome have a relatively poor prognosis for recovery of bowel and bladder function.
Cauda Equina Syndrome
Cauda equina syndrome (CES) results from an injury to the nerve roots emanating from the conus medullaris, at the level of L2 and below. This is not a type of SCI. Common etiologies are large herniated disk, burst fractures, epidural hematomas, compressive tumors, and ankylosing spondylitis. Unlike conus medullaris syndrome, CES tends to be asymmetrical and display only lower motor neuron symptoms.
In the setting of an acute CES, patients may present with radicular pain, patchy paralysis, saddle anesthesia, and arreflexia involving more than one nerve root. During initial evaluation, it is critical to evaluate patients for sphincter disturbances, particularly urinary retention as well as urinary and fecal incontinence.41 Presence of urinary retention at any time during evaluation of a patient has 90% sensitivity for the diagnosis of CES. Timing of surgical decompression in CES remains controversial; some studies showed no benefit with early surgical intervention.42,43 However, surgical decompression within 48 hours of onset of symptoms is recommended to improve the potential for recovery of bowel and bladder function.44
Spinal Shock
Spinal shock (see also Chapter 2) has been used to describe two very different phenomena following SCI. For instance, the term has been used inaccurately to describe neurogenic shock resulting from loss of sympathetic tone and bradycardia and hypotension in the trauma patient. Neurogenic shock is different from hypovolemic shock and should always be distinctly identified in an SCI patient with evidence of internal or external bleeding. Treatment includes judicious use of fluid resuscitation, pressors, and elevation of the patient’s legs.
True spinal shock refers to the temporary loss or depression of all or most spinal reflex activity below the level of the injury. In patients with complete SCI, spinal shock will precede a period of gradual progression to hypertonia, hyperreflexia, and spasticity. Ditunno et al.45 proposed a four-phase model describing the evolution of spinal shock over time. Phase 1, which occurs during the first 24 hours, is characterized by complete loss or depression of all reflexes below the level of injury. This phenomenon is thought to result from disruption of signaling between cerebral and spinal cord neurons. Phase 2 occurs from 24 to 72 hours postinjury and is marked by the return of polysynaptic reflexes, such as the bulbocavernosus reflex. This reflex can be monitored by performing a rectal examination and either pinching the glans penis or tugging on the Foley to observe involuntary contraction of the rectal sphincter. The later phases 3 and 4 are the hallmark of interneuron and lower motor neuron sprouting. Phase 3 may occur within 4 weeks and is mediated by axon-supported synaptic growth, whereas phase 4 occurs in the following weeks to month with soma-supported synaptic growth. These phases are characterized by a progression to hyperreflexia and spasticity.
 Conclusion
Conclusion
Over the years, a variety of classification systems have been created for the evaluation of SCIs. The ASIA classification system is currently the most widely used. It takes into account the patient’s neurological exam in terms of motor, sensory, and sacral function; it also evaluates the patient’s functional status. The ASIA system has a good interrater validity and reliability and is a good predictive tool of functional outcomes of SCI patients.
Pearls
 The initial neurological evaluation of SCI patients is critical because it dictates the acute management and determines the long-term prognosis.
The initial neurological evaluation of SCI patients is critical because it dictates the acute management and determines the long-term prognosis.
 ASIA/IMSOP standards are described in Fig. 6.1 and are commonly used to describe SCI.
ASIA/IMSOP standards are described in Fig. 6.1 and are commonly used to describe SCI.
 Some common forms of incomplete SCI syndromes are described in the chapter and include central cord syndrome, Brown-Séquard syndrome, anterior and posterior cord syndrome, conus syndrome, and cauda equina syndrome.
Some common forms of incomplete SCI syndromes are described in the chapter and include central cord syndrome, Brown-Séquard syndrome, anterior and posterior cord syndrome, conus syndrome, and cauda equina syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


