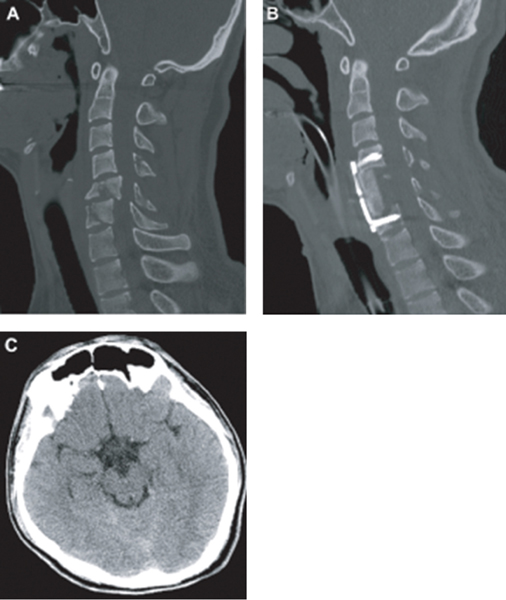
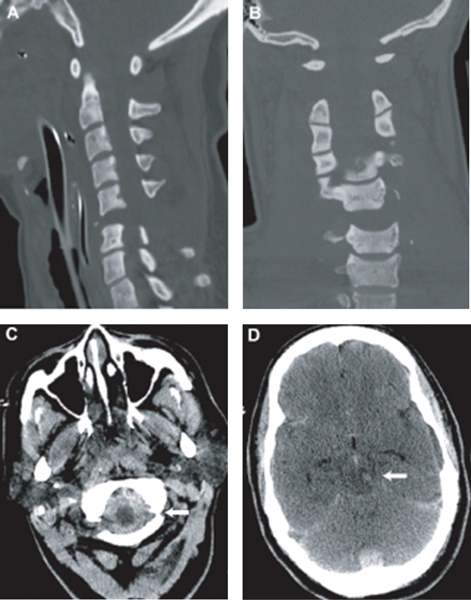
9 Key Points 1. Many cases of SCI, especially cervical cord injury, are accompanied by concomitant brain injury. 2. Individuals with SCI who also incurred mild or moderate TBI may be expected to have less functional recovery than those with SCI alone. 3. Concurrent SCI and TBI remain underdiagnosed and understudied. More attention to this complication of SCI is needed to improve both acute treatment and rehabilitation. Traumatic spinal cord injury (SCI), and especially cervical SCI, is often accompanied by traumatic brain injury (TBI), although the reported coincidence varies widely between studies. A recent report of TBI complications in military personnel in Iraq reported a significant incidence of SCI (9.8%) along with other multiple traumas.1 According to the Spinal Cord Model Systems dataset, 28.2% of SCI patients have at least a mild brain injury with loss of consciousness, whereas 11.5% have a TBI severe enough to demonstrate cognitive or behavioral changes.2 The complications associated with this “dual-diagnosis” are well known in the rehabilitation setting3 but may be underdiagnosed.4 A recent study by Macciocchi et al.5 estimated that 60% of SCI patients had at least mild TBI, and a prior retrospective study from this same center showed a substantial loss of recovery associated with dual diagnosis versus SCI alone.6 Identification of potential injury to the spine is critical in the evaluation of patients with blunt traumatic multisystem injuries. Depending on the mechanism of injury, the majority of patients with the dual diagnosis of TBI and SCI have injury to the cervical spine. However, assessment of the cervical spine in trauma victims presents specific challenges. Injuries in other systems can mask cervical column pain or discomfort, and obtundation from closed head injury or sedation limits the ability to perform an adequate clinical exam. Failure to recognize cervical injuries can have disastrous consequences. Few conditions are as devastating as cervical SCI and quadriplegia. Early diagnosis and acute management of identified or potential cervical spinal column injury and SCI are therefore essential in the treatment of the trauma patient. The epidemiology of SCI has been studied in more detail than that of spinal column injuries. Cervical spine injury occurs in ∼ 2 to 3% of patients with blunt trauma who undergo imaging studies.7 The incidence of SCI in the United States is estimated to be between 40 and 50 cases per million people per year, giving rise to 12,000 new cases per year.8 The global incidence of SCI has not been precisely studied, but estimates range from 10 to 83 cases per million population annually.9 Cervical SCI is more frequent than thoracic and lumbar injuries. Furthermore, there has been a small relative increase in the proportion of cervical SCIs, from 54.5% between 1973 and 1979 to 56.5% between 2000 and 2003.10 Individuals with SCI who also incurred mild or moderate TBI will have less functional recovery than those with SCI alone. This hypothesis is based upon both clinical consensus and limited practice-based and epidemiological evidence. Specifically, individuals who sustained a documented or undocumented mild to moderate TBI in addition to an SCI would evidence impaired cognitive function at admission, achieve smaller functional gains during rehabilitation, and require longer periods of rehabilitation than peers who sustained only an SCI. The cognitive and emotional sequelae of TBI hold the potential to adversely affect learning and skills acquisition and should, therefore, be assessed in relation to impact on the rehabilitation process. A retrospective, single-institution study6 has established that patients with SCI and comorbid TBI evidence smaller functional gains with rehabilitation. These results point to the importance of accurate and timely diagnosis and treatment of SCI in the setting of TBI. This chapter reviews the phenomenon of dual diagnosis, focusing on the cervical spinal cord, and discusses the challenges in diagnosis and management. TBI is defined as damage to brain tissue caused by an external mechanical force as evidenced by medically documented loss of consciousness or posttraumatic amnesia (PTA) due to brain trauma or by objective neurological findings that can be reasonably attributed to TBI on physical examination or mental status examination. Within TBI, there is a broad spectrum of processes that account for brain injury (i.e., concussion, epidural hematoma, subdural hematoma, parenchymal contusion, diffuse axonal injury, etc.). Similarly, SCI is defined as damage to the spinal cord caused by an external force. This injury would manifest itself in loss of motor or sensory function attributable to the level of SCI. The initial acute injury sustained during the inciting event can cause cord contusion, hematoma formation, or continued compression of the spinal cord. Various mechanisms can lead to such injury and have been well described by various groups, such as the AO Fracture Classification System (www.aofoundation.org). A patient is defined as having a dual injury diagnosis if he suffers from both of the foregoing injuries. In looking at the world’s largest database of SCI and TBI (the Spinal Cord Injury Model Systems of Care and the Traumatic Brain Injury Model Systems of Care, www.nscisc.uab.edu) there are common themes within these two patient populations: both have average ages in the 30s, the majority are male, and motor vehicle accidents and falls are the top causes of injuries. In the SCI population, the majority of patients have cervical injuries (50.7%), followed by thoracic (35.1%) and lumbosacral injuries (11%). Figures 9.1 and 9.2 illustrate two representative clinical cases of concomitant TBI and SCI. A complete and detailed initial physical exam in a patient with dual diagnosis is crucial because further progression or improvement of neurological deficits can determine the management course (i.e., surgical vs conservative) as well as the prognosis. In patients with suspected head injury, an admission computed tomographic (CT) scan of the head to evaluate for surgical lesions is important. While there is a tendency to focus on injuries to the head, injuries to the spinal cord should not be overlooked. In these patients, cervical spine injury must be assumed, and adequate radiographic images of the cervical spine should be obtained. Imaging should show all levels from the occiput to T1. If this cannot be achieved with plain radiographs, a CT scan with sagittal and coronal reconstructions should be obtained. Although this can exclude fractures or dislocation, magnetic resonance imaging (MRI) or dynamic fluoroscopy is required to rule out ligamentous injury. In awake patients, motor, sensory, reflex, and autonomic components should be thoroughly evaluated and the deficit levels noted. The spine should be palpated at each level, noting any tenderness, “step-off” deformities, or swellings. Fig. 9.1 This 22-year-old male was walking in the street during a windstorm. A billboard fell on the back of his head, resulting in loss of consciousness and hyperflexion of his cervical spine. He was intubated at the scene. His level of consciousness improved after resuscitation, but he was found to have a complete spinal cord injury with C5 level. (A) Admission midsagittal computed tomographic (CT) scan showing fractured C5 and C6 vertebral bodies and posterior elements. The posterior part of the C5 vertebral body is dislocated posteriorly within the spinal canal. (B) Postoperative midsagittal CT scan showing reduction and fusion of fracture deformity, done 48 hours after admission. (C) Admission brain CT scan at the level of the basal cisterns. The brain CT scan did not show any abnormality, consistent with severe concussion. Fig. 9.2 This 42-year-old female was a pedestrian hit by an SUV at high speed. The Glasgow Coma Scale score at the scene was 3, and she had absent vital signs. Bystanders at the scene and emergency medical service revived her. She did not regain any neurological function after resuscitation and required inotropic support for neurogenic shock. (A,B) Sagittal and coronal computed tomographic (CT) scan of the cervical spine showing severe fracture dislocation and distraction at C5-6. (C) CT scan of the brain showing thick subarachnoid hemorrhage (SAH) at the craniocervical junction (arrow). (D) CT scan of the brain showing tight basal cisterns and hemorrhage within the brain stem (arrow). Because of the patient’s poor neurological status and evidence of brain stem injury, aggressive management was not instigated. After discussion with the family, life support was withdrawn. SCI can result in complete or incomplete neurological deficits. In complete SCI, both motor and sensory components are absent below the level of injury, down to the lowermost sacral segments (S4 and S5). Sensations are absent, with paralysis and flaccid muscle tone. However, there may be partial motor or sensation present at the level just caudal to the injury with complete neurological deficit below this level. Reflexes are absent acutely but may become hyperactive with time. Patients can present with urinary retention with absent rectal tone, and males can present with priapism. The loss of sympathetic tone may result in neurogenic shock, which is more common in SCIs above the T6 level. Complete injury above the C3-5 level may also result in phrenic nerve paralysis and respiratory failure. In incomplete injury, motor or sensory components may be partially preserved below the level of injury. Incomplete SCI has better prognosis for recovery of function, whereas only 1 to 2% of patients with complete SCI recover significant distal cord function. Spinal shock refers to the loss of somatic motor, sensory, and sympathetic autonomic function after SCI. Although the mechanism is unclear, it is thought to be due to loss of nerve conduction from deranged levels of electrolytes and neurotransmitters. The presence of spinal shock can cause significant confusion in the initial neurological assessment. The low blood pressure, if not treated aggressively, can also lead to secondary brain injury in patients with a dual diagnosis. The effect of spinal shock in regard to autonomic dysfunction, such as the bulbocavernosus reflex, and reflex dysfunctions may persists for days to weeks after SCI. However, the clinician should assume that its effects on the somatic motor and sensory examination have resolved by 1 hour after the initial injury. Numerous clinical assessment scales have been developed to evaluate SCI patients. All of these scales assess either neurological or functional capacities of SCI patients to gauge loss or gain of function over time with consistent interrater reliability. Although it is routine to obtain and record the Glasgow Coma Scale (GCS) score for TBI patients, standardized assessment of SCI is not consistently performed. The most commonly used scale is the one developed by the American Spinal Injury Association (ASIA) in conjunction with the International Medical Society of Paraplegia (IMSOP). This scale divides patients into five grades of neurological deficits. Grade A denotes complete injury with no motor or sensory function. Grades B through D denote incomplete injuries below the anatomical level of the insult. Grade B denotes preserved sensory but no motor function, Grade C denotes preserved motor function with muscle strength grade less than 3, and Grade D denotes preserved motor function with muscle strength grade 3 or greater. Grade E denotes normal motor and sensory function. Furthermore, functional assessment tools have been developed, such as the Functional Independence Measure (FIM).11 The FIM scores the patient’s ability to perform activities of daily living, such as eating, grooming, and toileting. Thus, recovery over time can be measured in both neurological and functional components by incorporating the ASIA/IMSOP scale with the FIM. This combination of assessment tools not only measures the patient’s neurological improvements over time but also incorporates improvements in functional capacities that may occur in the absence of neurological improvement. Every trauma patient subjected to significant mechanisms of injury is suspected to have a spinal injury until proven otherwise. Patients with cervical injuries are particularly vulnerable during transfer procedures due to the higher mobility of the cervical spine and the relative importance of the cervical spinal cord at risk. The absolute primacy of the “ABCs” cannot be overemphasized, especially in the setting of a TBI and an SCI. Adequate perfusion and oxygenation of injured brain and spinal cord are essential to optimize recovery. Even brief periods of hypoperfusion and hypoxia can trigger secondary injury mechanisms, which increase morbidity and mortality and decrease chances of neurological recovery.12 Complete immobilization of the entire spine from the onset is necessary to prevent further injury to an already damaged spinal column or cord. If endotracheal intubation is required, the cervical spine is maintained in a neutral position without extension by applying gentle in-line traction. These important measures, which are implemented guidelines in the Advanced Trauma Life Support (ATLS) protocols, have contributed to a reduction in the ratio of quadriparesis to paraparesis in patients with multisystem trauma.13 The restoration of systemic hypotension to normotension is now a recognized principle of the emergency management in SCI based on the recognition that there is vascular compromise of the injured cord by local microcirculatory events. Initial resuscitation consists of volume replacement with crystalloids and blood products if persistent bleeding is suspected. Hypotension from neurogenic shock is much less common than hypovolemia in trauma patients, even in those with SCI, and is considered only after adequate volume replacement has been achieved and potential sources of ongoing bleeding have been ruled out. Treatment of hypotension in this case involves vasopressor agents. Furthermore, prevention of hypoperfusion and hypotension is particularly important during surgical intervention, especially for other nonneurological injuries, such as orthopedic injuries, where intraoperative blood loss can be pronounced. We therefore recommend that nonurgent surgeries be postponed during the first week of the acute injury period to reduce the chance of secondary brain and spinal cord injuries. If urgent surgical intervention is necessary, specific instructions are to keep the mean arterial blood pressure above 80 mmHg or the cerebral perfusion pressure between 60 mmHg and 70 mmHg if an intracranial pressure monitor is present. The hemoglobin is also maintained at 10 g/dL to ensure adequate oxygen delivery. It is recommended that all TBI patients with an admission GCS score of less than 8 be subjected to intracranial pressure monitoring. We attempt to place ventriculostomy catheters in most patients so that the cerebrospinal fluid (CSF) can be evacuated to relieve intracranial pressure. Even if the patient is taken to surgery, such CSF diversion can be a temporizing measure before surgical decompression is completed. Additionally, such monitoring may serve as a way to assess intracranial pressure during nonintracranial surgical intervention (spine, orthopedic, vascular, etc.). The use of osmotic agents has demonstrated efficacy in TBI patients with intracranial hypertension.14 However, the use of mannitol or hypertonic saline in treatment of SCI has not been demonstrated. Current use of osmotic agents in addressing SCI has been anecdotal and limited to published case reports with limited outcome data. However, recent data from a rat model of cervical SCI showed that 5% hypertonic saline given periodically over the 8 hours following injury reduced MRI indices of hemorrhage and edema.15 Because hyper-tonic saline has been indicated for TBI,16 this treatment may be an example of one that would be useful for combined SCI/TBI. Currently, we recommend that osmotic agents be used to treat intracranial hypertension if needed and not be used solely for SCI because there is no measureable end point for cord-specific treatment. Thus, although preclinical studies suggest a potential role for hypertonic saline in the treatment of SCI, more clinical studies are needed. Intravenous corticosteroid therapy is the only pharmacological therapy currently used in the acute treatment of SCI. Two randomized, controlled trials, NASCIS II and NASCIS III, published in 1990 and 1997, respectively, have shown benefits in patients treated with methylprednisolone in the acute setting.17,18 A bolus of 30 mg/kg of methylprednisolone is given to patients seen within 8 hours of their injury. If the initial bolus is given within 3 hours, the treatment is continued with a continuous infusion of 5.4 mg/kg/h of methylprednisolone for 24 hours, whereas patients who received the initial bolus between 3 and 8 hours receive an infusion for 48 hours. The use of corticosteroids in the treatment of SCI has come under criticism since its inception.19 Some critics have suggested that the benefits claimed in the original studies were not clinically significant and are outweighed by the risks and complications of the high-dose corticosteroid treatment. However, expert panels have maintained their support of this treatment as a recommendation but not a guideline in the treatment of SCI.20 Patients with cervical SCI can benefit the most from gaining functional levels or be significantly more impaired by losing only one level. For example, maintenance or acquisition of C6 musculature provides a major increment in the functional status of quadriplegic patients, allowing them to transfer from bed, propel a wheelchair, and live independently. A gain or loss of level(s) in the setting of a mid- to lower-thoracic SCI is less likely to affect the final functional recovery and may therefore not warrant steroid treatment, especially in higher-risk patients. The use of steroids for SCI in the dual diagnosis patient is particularly problematic given the recent results of the CRASH trial.21 This mega trial demonstrated that patients receiving methylprednisolone for the treatment of TBI did worse. The TBI patients in this trial had severe TBI, defined as a GCS score of 8 or less. The study did not address the effect on patients with mild and moderate TBI. This raises the question of whether to treat a trauma patient with both SCI and TBI with steroids. Given the complexity and heterogeneity of these injuries in humans, small- and large-animal models of combined injury treated with steroids will likely provide information to guide future clinical trails. The timing of surgery for intracranial pathology is relatively straightforward in comparison to a spinal pathology. For an intracranial space-occupying lesion, such as a hematoma, surgical decompression is performed when the size of the lesion or CT characteristics meet the criteria for surgical intervention or medical therapy fails to address the rise in intracranial pressure. During cranial surgery, it is important to note the possibility of cervical or spinal injury and to keep the head in a neutral position in a cervical collar with spinal precautions. In patients with known spine fractures and instability, a fluoroscope can be helpful to evaluate the patient before, during, and after positioning. Likewise, if the patient is taken to the operating theater to address a spinal pathology, it is important note the intracranial pressure during surgery. If there is any concern regarding the intracranial pressure during surgery due to an intracranial lesion, an intracranial pressure monitor should be placed and measures taken to decrease the pressure (elevation of the head, osmotic therapy, hyperventilation, etc.) if it is indeed elevated during surgery. For cervical injuries, surgical fusion is required when injuries to the cervical spine result in instability. Classification systems like the SLIC system can be used to determine the need for fusion.22 With the SLIC system, injuries with a score of 5 or more are all treated surgically, whereas those with a score of 3 or less are treated nonsurgically. A score of 4 is considered equivocal. There are two types of surgical approaches to the spine, anterior and posterior. Anterior approaches are best suited in the setting of mechanical failure of the anterior two columns, such as in vertebral body burst fracture, or when the spinal cord is compressed by elements located anteriorly, such as disrupted herniated disks. Posterior approaches can be used when no compressive elements are present anteriorly, when the posterior elements are severely disrupted, or when reduction from an anterior approach is not feasible or has failed. Severe injuries, such as in translation/rotation injuries, or when the three columns are disrupted, sometimes warrant both anterior and posterior procedures, referred to as 360 degree procedures. Thoracolumbar injuries follow a similar treatment algorithm. Classification with the TLICS can be used to determine the need for surgical stabilization.23 TLICS and the Magerl/AO classification were demonstrated to have good correlation for surgical management prediction.24 With the TLICS system, injuries with a score of 5 or more are treated surgically, whereas those with a score of 3 or less are treated nonsurgically. A score of 4 is equivocal. The surgical procedure is determined by the following general principles: (1) incomplete neurological injury requires an anterior procedure if compression from anterior spinal elements is present; (2) posterior ligamentous complex disruption requires a posterior procedure; and (3) combined incomplete neurological injury and posterior ligamentous complex disruption generally require a circumferential stabilization procedure. The role and timing of surgical decompression in the setting of an SCI remain among the most controversial topics in spinal surgery, due to the lack of well-executed randomized, controlled trials.25 Early decompression and stabilization of spinal column fractures allow early mobilization to prevent complications, such as pulmonary and urinary infections, decubitus ulcers, and deep vein thrombosis. Neurological worsening associated with persistent spinal cord compression by disk, bone fragments, or dislocated elements is a widely accepted indication for early surgery. Although it seems intuitive that early decompression after SCI may improve neurological recovery, the question remains for the most part unanswered. It has been thought in the past that early spinal surgery increases morbidity and mortality in patients with SCI.26 However, modern techniques of spine surgery as well as advances in neurocritical care and neuroanesthesia have allowed these patients to undergo early surgery without a significant increase in complications.27 Animal and radiological studies have shown that mechanical factors, such as persistent compression, are important in the pathogenesis and recovery from SCI.28,29 Moreover, several prospective series suggest that early decompression can be performed safely and may improve outcome.27,30,31 A meta-analysis of published clinical studies up to the year 2000 suggested that early decompression within 24 hours resulted in better outcomes compared with delayed decompression and conservative management.32 Based on the foregoing assumptions, early decompression and stabilization may provide patients with SCI an optimal window neurological recovery, early mobilization, and rehabilitation. The most commonly used antiepileptic drug, phenytoin, is given routinely for seizure prevention during recovery from TBI.33 This drug has been shown to be neuroprotective, in addition to (or in part because of) reduction of seizures and hyperexcitability. Previous studies have demonstrated the lack of efficacy in preventing long-term seizures with phenytoin use.34 Additionally, the side effects of antiepileptics, including motor and cognitive dysfunction, could potentially retard recovery and rehabilitation. Therefore, short-term use of phenytoin is advised. However, the issue is complicated by the presence of preclinical data suggesting that agents like phenytoin that reduce Na+-channel permeability may have a neuroprotective effect in both SCI35 and TBI. Neuropathic pain is often a side effect of SCI. In a recent animal study, a commonly used pain medication, morphine, was shown to impair recovery of locomotion in a rat thoracic SCI model.36 It is important to note that drugs used to reduce neuropathic pain after injury, such as gabapentin and opiates, may retard recovery and the cognitive and plasticity processes needed for rehabilitation after dual injuries and should be used only as necessary. And yet these drugs may also be neuroprotective. This points out the need for good animal models of SCI plus TBI to address some of these issues. Such models are conspicuously lacking. The dual diagnosis of concomitant brain injury and SCI has only recently been recognized as a distinct diagnostic entity despite its high prevalence in the traumatic setting. Thorough clinical and radiographic evaluation of the spine, particularly the cervical spine, is essential following blunt head trauma. In the setting of SCI, early diagnosis and treatment are necessary to prevent neurological deterioration. This may not always be possible in the dually diagnosed patient with a severe TBI and active neurocritical care issues, such as elevated ICP. Although questions remain regarding optimal timing of surgical treatment and medical management of SCI, it is likely that the evolving multidisciplinary approach will lead to improved treatment, recovery, and outcome for patients with concomitant TBI and SCI. Pearls
Concomitant Traumatic Brain Injury and Spinal Cord Injury
 Dual Injury Definition
Dual Injury Definition
 Clinical Assessment
Clinical Assessment
Complete versus Incomplete Spinal Cord Injury
Spinal Shock
ASIA/IMSOP Clinical Assessment Scale
 Management
Management
Acute Interventions
Role and Timing of Surgical Intervention
Subacute Interventions
 Conclusion
Conclusion
 Every trauma patient subjected to significant mechanisms of injury is suspected to have a spinal injury until proven otherwise.
Every trauma patient subjected to significant mechanisms of injury is suspected to have a spinal injury until proven otherwise.
 Early spinal decompression (< 24 hours) may provide SCI patients an optimal window of neurological recovery, early mobilization, and rehabilitation.
Early spinal decompression (< 24 hours) may provide SCI patients an optimal window of neurological recovery, early mobilization, and rehabilitation.
 Surgical parameters in concomitant injury are mean arterial pressure > 80 mmHg, cerebral perfusion pressure 60 to 70 mmHg, hemoglobin > 10 g/dL.
Surgical parameters in concomitant injury are mean arterial pressure > 80 mmHg, cerebral perfusion pressure 60 to 70 mmHg, hemoglobin > 10 g/dL.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine
