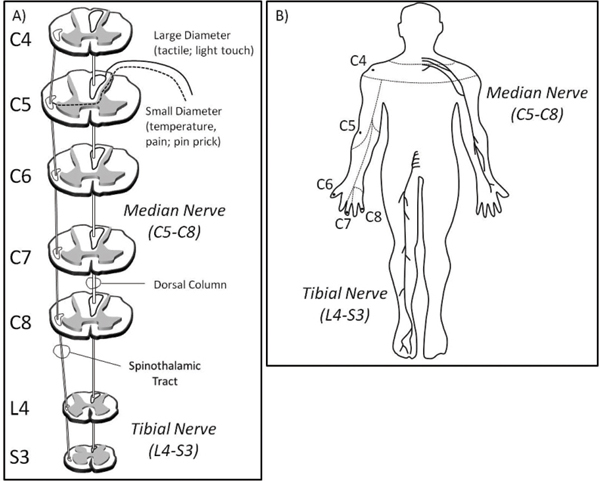
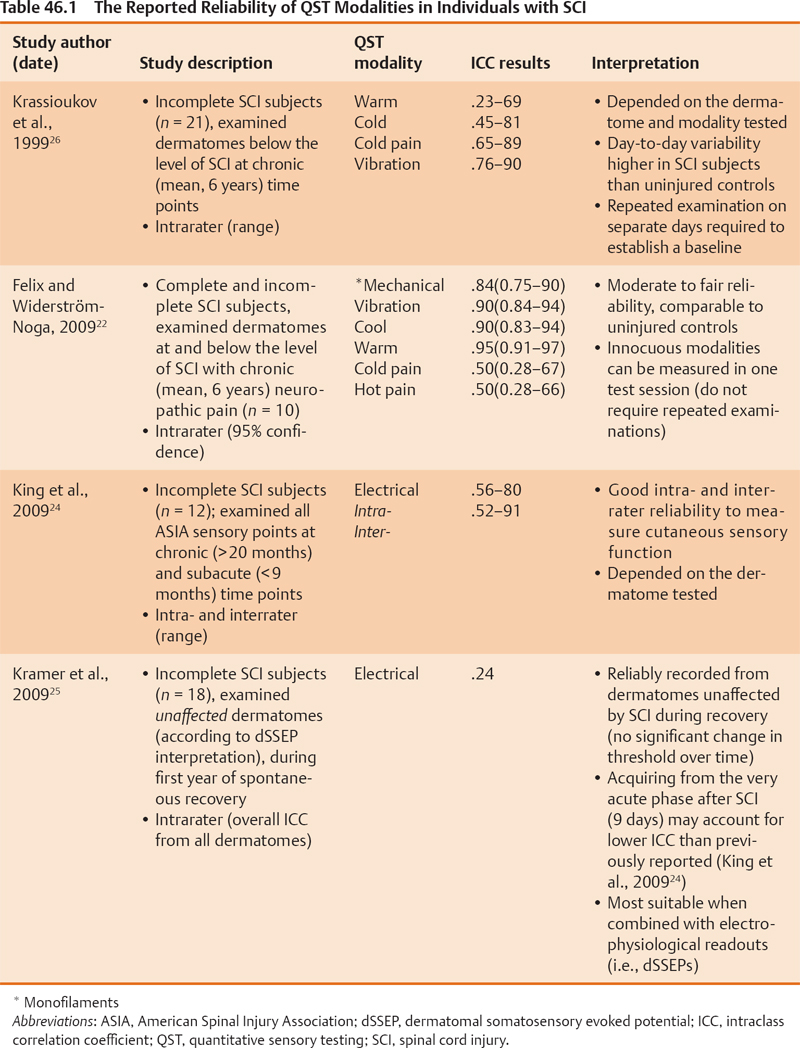
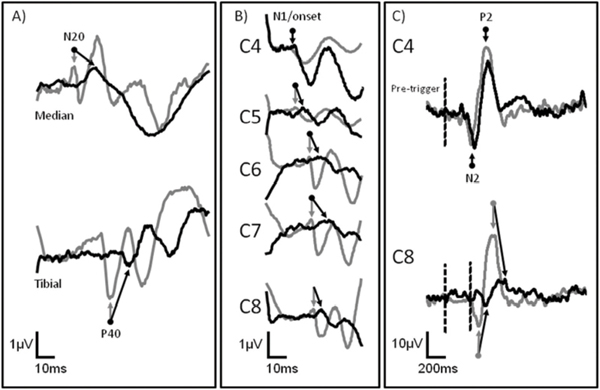
46 Key Points 1. Clinical light touch and pinprick testing disclose changes in the perception of sensation with limited insight into the physiology of sensory pathways. 2. Quantitative sensory testing complements sensory testing due to increased sensitivity and responsiveness in the assessment of sensory pathways. 3. Segmental sensory evoked potentials may indicate changes in the conduction of large- and small-diameter pathways that are missed by clinical testing. The assessment of somatosensory deficits after spinal cord injury (SCI) is an important component of the clinical examination. Impairments to different sensory modalities (e.g., proprioception, touch, and temperature) may vary depending on the disruption of ascending fibers in the spinal cord, range from diminished or absent sensation to complex hypersensitivity or hyperalgesia, and change during the course of spontaneous neurological recovery or secondary neurological deterioration (i.e., syringomyelia). The preservation of sensation after SCI is also considered a requisite for achieving desirable motor outcomes during rehabilitation and is related to the prediction of future performance of activities of daily living (ADLs), quality of life (QoL), and functional independence. Additionally, the plasticity of the somatosensory system may be a neural target for rehabilitation and therapeutic strategies that promote repair and regeneration, but which may also result in detrimental aberrant sprouting underlying neuropathic pain. Given the importance of closely monitoring sensory function after SCI for diagnosis, prognosis, and recovery (spontaneous or therapeutically derived), developing and implementing valid and reliable sensory measures that are responsive to subtle changes in afferent neurophysiology and related to functional outcomes are priorities of clinicians and researchers. This chapter focuses on the three main pillars of the clinical assessment of somatosensory function after SCI: (1) the American Spinal Injury Association (ASIA) Impairment Scale (AIS), (2) quantitative sensory testing (QST), and (3) electrophysiological recordings. The validity, reliability, and responsiveness of these measures to detect deficits in the spinothalamic tract and dorsal column are discussed in relation to the stable neurological condition (chronic SCI) and during spontaneous neurological recovery (transition from acute to chronic SCI). Also discussed is the utility of the outcomes measured during the course of a clinical trial in SCI to assess the effectiveness and potential side-effects of intervention. The classification of SCI by the AIS has undergone years of intensive study to examine psychometric properties1–11 and the responsiveness to spontaneous neurological recovery.12–17 Adopted by both the clinical and the research communities worldwide as a relatively undemanding test of basic motor and sensory function after SCI, the AIS has served as a stratification tool and primary outcome measure in all of the randomized clinical trials in SCI to date.18,19 The sensory component of the AIS is based on the theoretical framework that dermatomes with defined boundaries in the periphery represent individual spinal segments anatomically organized into large- (light touch) and small-diameter (pinprick) fiber functions, which ascend in the dorsal column and spinothalamic tract, respectively (Fig. 46.1). The decussation points of these afferent fibers into the ventrolateral spinothalamic tract (i.e., small-diameter fibers, on entry into the spinal cord segment), and dorsal column (i.e., large-diameter, dorsal column nuclei in the brain stem) represent the key anatomical difference accounting for the dissociation of light touch and pinprick sensory impairments after SCI. The outcomes of sensory examination are at first integral in diagnosing and predicting long term the severity (i.e., sensory preservation at S4–5), neurological level, and functional deficits associated with SCI, and later, the planning of an appropriate rehabilitation program. The reliability of AIS sensory outcomes is well supported in the literature by several large and statistically powerful studies. In general, studies examining the test–re-test (i.e., intrarater) and interrater reliability of light touch and pinprick scores in the chronic, stable SCI condition demonstrate intraclass correlations that exceed the minimal requirements of clinical standards and strong interrater agreement. However, the reliability of light touch and pinprick may be influenced by several factors, including when the examination is performed after SCI, characteristics of the individual being examined (i.e., age), severity of SCI, and formal training of the examiner. The two primary outcomes of the sensory component of the AIS (completeness of injury and cumulative light touch and pinprick sensory scores) are both subject to change during the course of spontaneous neurological recovery. First, the return of anal sensation (fourth and fifth sacral spinal cord segment) marks an important clinical event by which an individual spontaneously converts from a complete (AIS A) SCI to an incomplete (AIS B–D) SCI. So termed “AIS grade conversion” can be observed most prominently within the first year of SCI,13,15,19,20 but it has been reported infrequently between 1 and 5 years after injury.21 Recovering sensation in dermatomes near the level of lesion may also shift the neurological level of SCI caudally, thereby improving function in a segmental fashion. Unfortunately, conversion from complete to incomplete SCI occurs rather infrequently spontaneously or due to therapeutic intervention, and the spontaneous recovery of sensory scores (light touch and pinprick) is also quite small. These findings should be interpreted with caution, to illustrate the insensitivity of an ordinal measurement scale incapable of tracking changes within “impaired” dermatomes (i.e., hyper- and hyposensitive) rather than unchanging sensory deficits after SCI. Fig. 46.1 (A) The segmental and longitudinal anatomical organization of ascending pathways in the spinal cord. (B) The distribution of key American Spinal Injury Association (ASIA) Impairment Scale (AIS) dermatomes in the upper limbs examined for light touch and pinprick sensation after spinal cord injury. The median (upper limb) and tibial (lower limb) nerves examined by conventional sensory evoked potentials are also shown. The primary goal of implementing QST as an adjunct to the standard examination of sensory function is to improve the overall sensitivity and clinical meaningfulness of sensory outcomes after SCI. Specifically, QST methods aim to (1) detect underlying deficits in dermatomes with “normal” sensation, and (2) distinguish differences in dermatomes with “impaired” sensation. QST methods still require that the individual being tested report the sensation to an examiner but differ from the AIS by allowing a continuous scaled outcome to mark distinct sensory thresholds (i.e., perception and pain). Several different QST modalities have been tested in individuals with SCI (Table 46.1).22–28 Typically, thresholds are achieved by increasing the intensity of stimulation at a set rate from baseline until the individual verbally reports a change in sensation (i.e., method of limits). QST outcomes have generally been found to correlate with electrophysiological outcomes and the AIS examination and appear more sensitive to sensory impairments rostral to the neurological level of SCI. However, most QST measures have not been employed by clinicians due in part to practical limitations (i.e., time-consuming procedure and expensive equipment), but also a lack of sufficient data regarding their utility to reliably distinguish differences in dermatomes with clearly impaired sensation (i.e., hypersensitive vs hyposensitive) and track changing sensory deficits during spontaneous recovery.29 To address many of the concerns about existing QST measures, electrical perception threshold (EPT) testing was introduced to SCI in 200628 and has been well studied regarding psychometric properties in both control subjects and individuals with chronic SCI. This assessment technique, which requires a subject to report perception of an increasing electrical stimulation from self-adhesive surface electrodes positioned on key areas of each dermatome, requires minimal technical experience, uses relatively inexpensive equipment, and appears sensitive to varying degrees of sensory deficits after SCI. Independent groups have shown the reliability of this measure in chronic SCI, and responsiveness studies in acute SCI are under way. Proposed as a measure of dorsal column function,29 the major drawback of EPT at present is that stimulation is not physiologically specific; rather, it may broadly activate both small-and large-diameter fibers in the periphery. Largely considered a complementary assessment to the AIS examination of SCI severity, the inclusion of electrophysiology as a standard outcome measure intends to provide a more sensitive and objective index of spinal cord function. Conventional somatosensory evoked potential (SSEP) outcomes (i.e., amplitude and latency) are most commonly examined by upper- and lower-limb stimulation of mixed nerves in the periphery using square-wave electrical pulses to record the function of the dorsal column. The characteristic waveforms of SSEPs recorded from scalp electrodes following peripheral electrical stimulation of the median and tibial nerves (upper/lower panel) are shown in Fig. 46.2A. After incomplete SCI (black line), prominent negative and positive peak potentials (N20, median nerve; P40, tibial nerve) are marked at increased latencies and decreased amplitudes compared with neurologically intact subjects (gray line). In general, the outcomes of electrophysiological assessment improve the prediction of long-term functional outcomes based on AIS preservation of sensation in S4-5 alone30 and are clinically very useful to detect SCI in unconscious individuals. In theory, implementing electrophysiology during the first year after SCI also allows a greater understanding of the mechanisms involved in spontaneous recovery (i.e., remyelination and/or regeneration). However, the responsiveness of conventional SSEPs during spontaneous recovery has only recently been studied in a large and statistically powerful cohort of individuals with SCI.13,17 In general, it seems that, although severely deteriorated SSEPs may undergo minor recovery of delayed latency and reduced amplitude, these changes do not correspond with the extent of the functional improvement typically achieved during spontaneous recovery. Electrical stimulation of individual dermatomes using the same electroencephalographic (EEG) methodological principles and protocol as for conventional SSEPs provides a more precise segmental neurophysiological assessment of posterior spinal cord innervation (dorsal root entry and ascending dorsal column conduction). Termed dermatomal SSEPs (dSSEPs), this approach shares the advantages of the AIS examination to record segment-by-segment sensory outcomes above, at, and below the level of lesion, and conventional electrophysiological outcomes to assess sensory function relatively independent of examiner or examinee interpretation and bias (Fig. 46.2B). In the example shown in Fig. 46.2B, a dSSEP is recorded in both an individual with SCI (i.e., above the level of the lesion) and a neurologically healthy control subject at normal N1/onset latencies. As the stimulation site moves caudally (from C4 to C8), the dSSEP recording latency of the prominent N1 component is increased greater than two standard deviations of normal control values in the individual with SCI, indicating impaired spinal conduction at and below the level of SCI. The reliability of this technique recorded from cervical dSSEPs has been demonstrated in neurologically healthy control subjects and recently in individuals with chronic-stable SCI.25,29 Fig. 46.2 (A) Conventional mixed nerve somatosensory evoked potentials (SSEPs), (B) cervical (C4-8) dermatomal SSEPs (dSSEPs), and (C) cervical contact heat evoked potentials (CHEPs) recorded from neurologically intact control subjects (gray), and after incomplete spinal cord injury (SCI) (black). Arrows indicate the marking of the most prominent and reliable component of the waveforms. The increased latency and decreased amplitude of sensory evoked potentials are characteristic of spinal conductive deficits after SCI; however, only a segmental approach is useful to track changes within different levels above, at, and below the injury.
Somatosensory Function and Recovery after Spinal Cord Injury: Advanced Assessment of Segmental Sensory Function
 Sensory Examination According to the ASIA Impairment Scale
Sensory Examination According to the ASIA Impairment Scale
 Quantitative Sensory Testing
Quantitative Sensory Testing
 Neurophysiological Recordings of Sensory Function
Neurophysiological Recordings of Sensory Function
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Musculoskeletal Key
Fastest Musculoskeletal Insight Engine
