CHAPTER 36 Soft Tissue Coverage of the Elbow
INTRODUCTION
Difficult-to-manage soft tissue defects about the elbow occur as a result of trauma, infection, extravasation of chemotherapeutic agents, cutaneous ulceration or necrosis, and limb-sparing tumor surgery. Treatment options are many, and appropriate management requires careful consideration of all alternatives. Coverage choices may include primary closure, skin grafting, local cutaneous flaps, fasciocutaneous transposition flaps, island fascial or fasciocutaneous flaps, local or distant one-stage muscle or myocutaneous transposition, distant temporary pedicle flaps, and microvascular free tissue transfer. Historically, the reconstructive algorithm for providing elbow coverage has proceeded in a stepladder fashion, with the simplest procedures being used first to obtain coverage; however, with advancements in microsurgical techniques, the reconstructive ladder is often disregarded in preference for the surgical procedure, which provides the patient with the best form and function.
PATIENT ASSESSMENT
THE WOUND
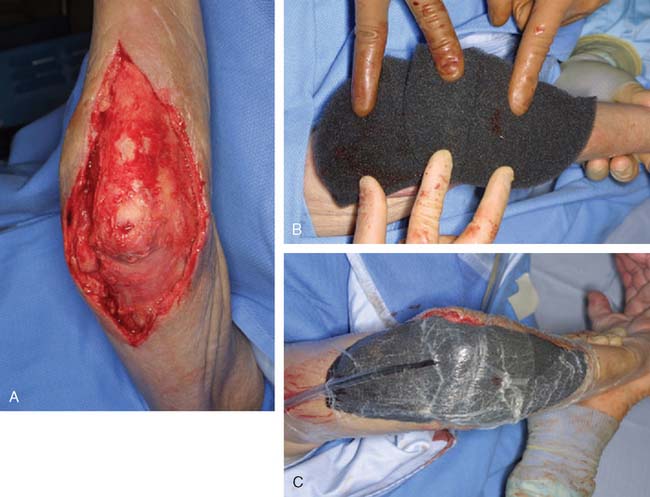
Most soft tissue defects benefit from early coverage. Advantages of expeditious closure include diminished edema, lower rates of wound infection, decreased scar formation and wound contraction, less pain, and enhanced upper limb function.65,69,106,110 Traumatic defects require thorough débridement and irrigation but often may be closed within 24 hours of injury, with salutary results.31,65 In contrast, complex wounds resulting from high-energy injury (electrical burns, crush, avulsion) and grossly contaminated wounds may require serial débridements and a delay to allow adequate assessment of tissue viability. Open packing, temporary coverage with biologic dressings or use of negative pressure therapy devices (vacuum-assisted closure [VAC]) may be required during this phase of management. VAC therapy has become an important adjunct in the management of major soft tissue injuries over the last 10 years. VAC therapy (V.A.C., KCI, San Antonio, TX) uses a polyurethane ether foam with a pore size of 400 to 600 mm, which is cut to size and applied to the wound with a transparent dressing to create an airtight seal. Controlled intermittent or continuous topical negative pressure is then delivered to the wound by incorporating a tube into the seal, and this is provided by a dedicated vacuum pump appliance. Exudate, edema, and bacterial load is reduced and the growth of granulation tissue is encouraged, and these properties make it a useful tool in any algorithm for soft tissue reconstruction because it has the potential to convert a difficult wound into one that may be closed or covered with a simpler technique. Its use is particularly important in two scenarios—when systemic comorbidity precludes a major lengthy reconstructive procedure; and when local wound factors, such as heavy contamination, necessitate multiple wound débridements before the definitive wound closure.3 In these situations, VAC therapy is not usually a substitute for surgery, but it offers a bridge until definitive surgery can proceed safely11 (Fig. 36-1).
RECONSTRUCTIVE METHODS
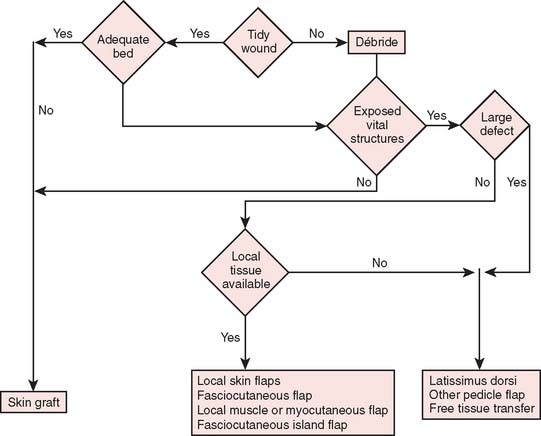
Reconstructive goals are to obtain optimal wound healing, restore function, and maximize aesthetic outcome, which should always be considered. Historically, the simplest method that provides adequate coverage and meets reconstructive needs is chosen. Thus, tension-free closure of an elbow defect by skin elevation and advancement is often performed. If tension-free closure is not possible, skin grafting can be considered for selected defects with well-vascularized tissue beds. Otherwise, the location, size, and tissue composition of a defect all in part dictate selection of an appropriate flap. Local flaps provide the best tissue match but are limited in size and can devascularize or weaken an already damaged limb. Larger defects and those that require composite tissues for primary reconstruction of tendon, muscle, bone, or sensibility demand consideration of other methods, including local island fascio-cutaneous flaps, local or distant pedicle muscle, myocutaneous transfer, and free tissue transfer. An algorithm for tissue coverage is useful in preoperative planning (Fig. 36-2).
SKIN GRAFTING
Indications
Skin grafting may be indicated for any defects with an acceptable bed. Exposed structures that will accept a graft include subcutaneous tissue, paratenon, and muscle. Other tissues, such as exposed bone, joint, tendon, and nerve, may be covered temporarily by graft used as a biologic dressing but will not support a graft for permanent coverage. Beds containing tissues of questionable viability, chronic granulation tissue, or frank infection must be appropriately débrided. Bacterial contamination, accumulation of serum, and a small amount of capillary bleeding are not contraindications for grafting. Tidy wounds that contain fewer than 105 bacteria per gram of tissue or that allow xenograft adherencewithin 24 hours allow successful skin grafting.27,52,61,93,107 Skin grafts are contraindicated in areas that are exposed to repetitive trauma or that lie over osseous prominences. In addition, skin grafting should be avoided in areas that may require secondary surgery for bone or nerve grafting. All split-thickness grafts will undergo some component of contracture over time; thus, if these grafts are placed over large areas of the antecubital fossa or olecranon, there is a risk of limitation in elbow motion. To improve durability, a collagen-glycoaminoglycan biodegradable matrix (Integra, Plainboro, NJ) can be grafted first to create a neo-dermis, then a split-thickness skin graft can be applied subsequently to improve durability of the graft.18
Technique
Small defects in areas where wound contracture is undesirable may be optimally covered with a full-thickness graft harvested from the inguinal crease. The thin, lax skin in this area provides excellent graft material and allows closure of the donor site with an inconspicuous linear scar. Split-thickness grafts that are harvested with a power-driven dermatome are used to cover larger areas. Any area may be used as a donor site; usually the proximal thigh is chosen because of its relatively flat contour and generally concealed location. The thickness of such grafts can range from 0.012 to 0.015 inch. Older adults and children with thin dermis should receive 0.012-inch thick graft to avoid full thick skin loss at donor site. A meshed graft allows expansion and is indicated for large or draining defects. The donor site is dressed with a plastic vapor-permeable dressing or fine mesh gauze, and exudate is removed from beneath the donor site with a syringe, as needed, or the gauze is dried with a heat lamp.45,96 The graft is secured with chromic sutures or stables and covered with Xeroform or some other form of nonadherent occlusive dressing. Immobilization of the graft during the early stages of healing is essential for complete graft survival and may be accomplished with either the use of a bolster or a VAC sponge. A cotton bolster may be created with gauze and saline-soaked cotton balls. Nylon sutures secured to the margin of the graft are tied over the cotton, firmly compressing the skin to its bed. The elbow is immobilized, and the graft is left undisturbed for 5 to 7 days. If VAC therapy is to be used to promote graft adherence the sponge is set to provide 75 mm Hg of constant suction for 5 days. This method has been shown to significantly improve skin grafting survival in a recent prospective randomized study.81
LOCAL FLAPS
Random Cutaneous Flaps
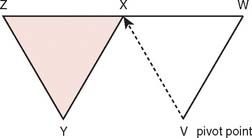
Often, small defects unsuitable for grafting may be covered by skin immediately adjacent to the primary defect. Such random cutaneous flaps carry their own blood supply through the dermal and subdermal plexuses and occasionally through a specific cutaneous artery oriented in the axis of the flap (axial flap). In general, random flap should be designed with a 1 : 1 length-width ratio. Such flaps are further categorized according to motion applied to the flap as it covers the defect; these flaps include transposition or translation flaps, advancement flaps, and rotation flaps.63 Transposition flaps are raised and moved laterally to an adjacent area, and the primary defect is closed by triangulating the defect and closing one angle of the triangle. The flap is then drawn as a parallelogram and raised (Fig. 36-3). Its pivot axis or critical line may be extended by lengthening or widening the flap or extending the cut parallel to the defect side (extension cut) or toward the defect (back cut).63 Closure of the secondary defect generallyrequires skin grafting. Other examples of transposition flaps include Z-plasties and rhomboid flaps (i.e., in the shape of an equilateral parallelogram). Rhomboid flaps are occasionally useful about the elbow when the defect may be excised or débrided to a rhomboid shape. A rhombus with 60- and 120-degree angles, the Limberg flap, is optimal, but other angles may be accommodated using Dufourmental’s method (Fig. 36-4).64 Such single rhomboid flaps have the disadvantages of creating tension at the flap tips and at the line of donor site closure, anatomic landmark displacement, and occasionally, cause difficulty in orientation for optimal scar appearance. A double-Z rhomboid may be a superior alternative for some defects (Fig. 36-5).21 Orientation of the long axis of the rhomboid to the relaxed skin tension line produces the least tension and “maximal cosmesis.”
Axial Fasciocutaneous Flaps
The above-mentioned random flaps are limited in their ability to cover large defects due to a poor random blood supply, limited mobility, and inadequate local skin elasticity. In contrast, the axial flap by inclusion of a known axial blood supply can allow safe extension of the length and narrowing of the base to improve coverage.79
The blood supply to the skin of the upper extremity was studied by Manchot71 more than a century ago and most recently by Le Huec and Liquois,59 by Salmon93a and Lamberty,56 and by others.94 A predictable pattern of cutaneous angiosomes exists that can be used in planning local flaps (Fig. 36-6). Blood from the named vessels supplying each of these regions may enter the skin from direct cutaneous vessels, musculocutaneous perforators, or fasciocutaneous vessels.19 These vessels form a plexus both subdermally and in deep fascia.108 Although forearm and elbow transposition flaps containing only subcutaneous tissue have been reported by Marty and colleagues, inclusion of deep fascia in transposition flaps improves viability.19,29,74,76 The bloodsupply to these areas of skin may come from multiple fasciocutaneous perforators, a single consistent and sizable pedicle, or multiple small branches from an underlying large vessel, the most dominant of which is the superior ulnar collateral artery.59
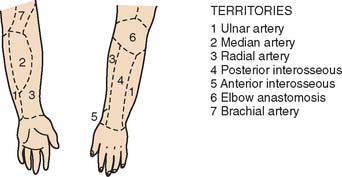
FIGURE 36-6 A reproduction of Manchot’s original diagrams of the forearm angiotomes.
(From Lamberty, B. G. H., and Cormack, G. C.: The forearm angiotomes. Br. J. Plast. Surg. 35:420, 1982.)
Fasciocutaneous transposition flaps may be raised from the medial arm based on the medial elbow anastomotic vessels (superior ulnar collateral artery and ulnar recurrent vessels), the lateral arm based on the terminal branches of the profunda brachii and their recurrent interosseous artery anastomoses, as well as forearm fasciocutaneous perforators from radial, ulnar, and anterior and posterior interosseous arteries.50,56,74,99 These perforators, arranged in four parallel rows, enter the forearm skin at regular intervals (Fig. 36-7).74 An example of such a flap is shown in Fig. 36-8, based on the posterior interosseous vessels and lateral elbow anastomotic blood vessels. Of all available forearm fasciocutaneous arteries, Lamberty and Cormack found that only the inferior cubital cutaneous artery, a branch of the radial recurrent artery, was sufficiently large and constant to reliably supply a large cutaneous area (most of the territory attributed to the median artery by Manchot) (see Fig. 36-6).56,57 Coverage of the olecranon is achieved reliably and easily with this tissue technique. In case of extensive trauma or reoperative surgery, it is advisable to use a hand-held Doppler device to confirmthe presence and extent of the vascular pedicle before raising the flap.
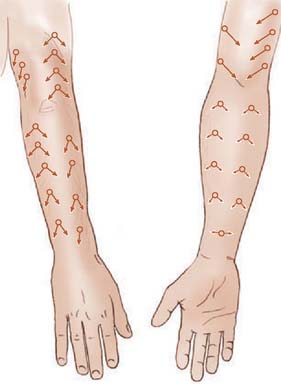
FIGURE 36-7 Location of cutaneous perforating vessels to the forearm.
(From Marty, F. M., Montandon, D., Gumener R., and Zbrodowski, A.: The use of subcutaneous tissue flaps in the repair of soft tissue defects of the forearm and hand: an experimental and clinical study of a new technique. Br. J. Plast. Surg. 37:95, 1984.)
Island flaps are the ultimate application of fasciocutaneous axial transposition flaps. Several upper extremity island flaps have been described in the past decade for use in orthograde or retrograde pedicle transposition or free tissue transfers. Island flaps are raised with a base consisting solely of a fully mobilized vascular pedicle, and they may include fascia, subcutaneous tissue, skin, and segments of vascularized muscle, bone, tendon, and cutaneous nerves, depending on the particular reconstructive needs. Some flaps used for elbow coverage are orthograde antecubital fasciocutaneous flap, radial forearm, ulnar forearm, and posterior interosseous flaps and retrograde medial and lateral arm flaps.* Their orthograde axial vascular pedicle allows the widest possible arc of rotation and present relatively little risk of circulatory embarrassment. Coverage of small and moderate defects on all surfaces of the elbow is possible without microvascular anastomoses and upper extremity mobilization.37
Antecubital Fasciocutaneous Flap
Described by Lamberty and Cormack,56,57 the antecubital fasciocutaneous flap is an axial-pattern, fasciocutaneous flap based on the inferior cubital artery. This vessel usually arises from the radial recurrent artery (17.5%) or from the radial artery proper (62.5%) and lies in the intermuscular septum between brachioradialis and flexor carpi radialis.70 It runs distally, paralleling the course of the cephalic vein in the superficial fascia. The vessels origin is 4 cm inferior to the midportion of the anterior interepicondylar line (Fig. 36-9). Venous drainage is provided principally by the cephalic vein.57 A 4:1 length-width-ratio flap may safely be raised.
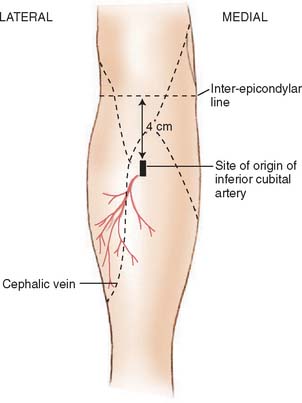
FIGURE 36-9 The distribution of the inferior cubital artery.
(From Lamberty, B. G. H., and Cormack, G. C.: The forearm angiotomes. Br. J. Plast. Surg. 35:420, 1982.)
Radial Forearm Island Flap
The cutaneous territory nourished by the radial artery includes the radial two thirds of the anterior forearm and its lateral aspect.56 A fasciocutaneous flap based on an orthograde (proximal) pedicle provides excellent elbow coverage of moderate-sized defects, including the posterior aspect of the region.2,24,28,35,63 Approximately 9 to 17 cutaneous perforators from the radial artery supply the skin, including musculocutaneous vessels proximally and fasciocutaneous vessels distally by means of a deep prefascial plexus and a subdermal plexus.105 Its chief advantages are its reliability, technical simplicity, and versatility, the results of its being a compound flap including fascia only, composite skin and fascia, vascularized brachioradialis, flexor carpi radialis, or palmaris longus tendon segment of the distal radius and its sensibility with lateral and medial antebrachial cutaneous nerves.26,37,82 Its arc of rotation allows coverage of all aspects of the elbow joint but is limited proximally by its point of rotation at the radial artery origin 10 cm below the elbow joint.105 The principal drawback of this method is the unsightly donor site, which requires split-thickness skin grafting unless a small area or fascia only is used.47 Although the size of the radial cutaneous territory may be as large as 15 by 25 cm, flaps generally no larger than 16 by 8 cm (usually smaller) are considered for elbow defects that measure 6 to 8 by 14 to 6 cm.24,26,56,104 A complete deep palmar arch is necessary for adequate perfusion of the hand by the ulnar and, occasionally, median arteries.
Other complications following radial forearm flap elevation can include decrease in radial nerve sensation, delayed healing at the skin graft site, decreased range of motion, and fractures of the radius following osteocutaneous flap elevation. Richardson found in a series of 100 patients that 13% experienced exposed tendons, 19% had delayed healing over the donor site and 32% had decrease sensation in the radial nerve distribution.91 Raising the flap with a portion of the radius has been associated with a high incidence of postoperative fractures.9,102,106
Attempts at limiting donor site morbidity have included modifications in flap elevation. The flap may be elevated as a pure facial flap, allowing for primary closure of the donor site.47,89 The flap may also be elevated suprafacially; this allows for fewer donor site complications with enhancement of skin graft take and elimination of tendon exposure.5,15,68 Further improvement to the donor site may be made by placing tissue expands before or following flap transfer to help with primary closure of the donor site.34,36,75 Also ulnar-based skin islands have been used to close the donor site.7,49 Despite donor site complications, the radial forearm flap has remained a workhorse flap for elbow coverage.
Technique
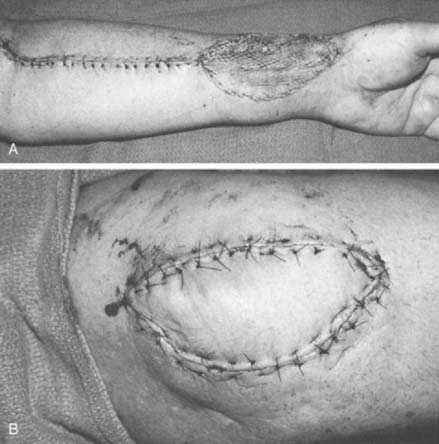
The course of the radial artery is marked with the help of a Doppler ultrasound probe, and a timed Allen’s test is performed to verify adequate distal circulation with the radial artery occluded. The donor skin area is outlined somewhat larger than the defect and as distal as possible to create a pedicle long enough to reach the elbow defect. It is centered over the radial artery and may include the cephalic vein. The flap is then raised from distal to proximal, protecting the superficial radial nerve distally but including the proximal cephalic vein and lateral antebrachial cutaneous nerve; this allows for the creation of a sensate flap. The margins are incised, including the antebrachial fascia. Distally, the radial artery is ligated and the flap, including the lateral intermuscular septum, radial vessels, and fasciocutaneous perforators, is raised as a proximally based island flap transposed to cover the elbow (see Fig. 36-9). Closure of the donor site requires split-thickness skin grafting (Fig. 36-10). Incomplete graft “take” is a common complication, but the risk may be minimized by preserving paratenon over distal tendons and by immobilizing the wrist and digits for several days after surgery.77,106 Further improvement in graft take may be accomplished by mobilization of the superficialis muscle belly over any component of exposed flexor carpi radialis tendon. We use a nonmeshed graft to improve donor site cosmesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree